Please watch the Video below to see real life examples of people who have been helped by this groundbreaking technology.”

Articles documenting MEG Imaging proof of IASIS effectiveness by UCSD research scientists are referenced below.
1. https://health.ucsd.edu/news/releases/Pages/2017-9-28-transcranial-electrical-stimulation-shows-promise-for-treating-mild-traumatic-brain-injury.aspx
2. https://doi.org/10.1080/02699052.2017.1327672
3. https://www.sciencedaily.com/releases/2017/09/170929093048.htm
Further proof of IASIS effectiveness is the fact that the VA and The University of California San Diego (UCSD) are now 2 years into a 4 year double-blinded, randomized research study called the VA/UCSD Merit Study of IASIS Technology applied to Vets with Multiple diagnoses, PTSD and mTBI issues. Here are the article links:
1. https://www.research.va.gov/about/funded-proj-details-fy2018.cfn?pid=574277
2. https://clinicaltrials.ucsd.edu/trial/NCT03244475
1. https://health.ucsd.edu/news/releases/Pages/2017-9-28-transcranial-electrical-stimulation-shows-promise-for-treating-mild-traumatic-brain-injury.aspx
2. https://doi.org/10.1080/02699052.2017.1327672
3. https://www.sciencedaily.com/releases/2017/09/170929093048.htm
Further proof of IASIS effectiveness is the fact that the VA and The University of California San Diego (UCSD) are now 2 years into a 4 year double-blinded, randomized research study called the VA/UCSD Merit Study of IASIS Technology applied to Vets with Multiple diagnoses, PTSD and mTBI issues. Here are the article links:
1. https://www.research.va.gov/about/funded-proj-details-fy2018.cfn?pid=574277
2. https://clinicaltrials.ucsd.edu/trial/NCT03244475
Transcranial Electrical Stimulation Shows Promise for
Treating Mild Traumatic Brain Injury
https://health.ucsd.edu/news/releases/Pages/2017-9-28-transcranial-electrical-stimulation-shows-promise-for-treating-mild-traumatic-brain-injury.aspx
https://www.sciencedaily.com/releases/2017/09/170929093048.htm
Transcranial Electrical Stimulation Shows Promise for Treating Mild Traumatic Brain Injury
September 28, 2017 | Scott LaFee
Using a form of low-impulse electrical stimulation to the brain, documented by neuroimaging, researchers at the University of California San Diego School of Medicine, Veterans Affairs San Diego Healthcare System (VASDHS) and collaborators elsewhere, report significantly improved neural function in participants with mild traumatic brain injury (TBI).
Their findings are published online in the current issue of the journal Brain Injury .
TBI is a leading cause of sustained physical, cognitive, emotional and behavioral problems in both the civilian population (primarily due to motor vehicle accidents, sports, falls and assaults) and among military personnel (blast injuries). In the majority of cases, injury is deemed mild (75 percent of civilians, 89 percent of military), and typically resolves in days.
But in a significant percentage of cases, mild TBI and related post-concussive symptoms persist for months, even years, resulting in chronic, long-term cognitive and/or behavioral impairment.
Much about the pathology of mild TBI is not well understood, which the authors say has confounded efforts to develop optimal treatments. However, they note the use of passive neuro-feedback, which involves applying low-intensity pulses to the brain through transcranial electrical stimulation (LIP-tES), has shown promise.
In their pilot study, which involved six participants who had suffered mild TBI and experienced persistent post-concussion symptoms, the researchers used a version of LIP-tES called IASIS, combined with concurrent electroencephalography monitoring (EEG). The treatment effects of IASIS were assessed using magnetoencephalography (MEG) before and after treatment. MEG is a form of non-invasive functional imaging that directly measures brain neuronal electromagnetic activity, with high temporal resolution (1 ms) and high spatial accuracy (~3 mm at the cortex).
“Our previous publications have shown that MEG detection of abnormal brain slow-waves is one of the most sensitive biomarkers for mild traumatic brain injury (concussions), with about 85 percent sensitivity in detecting concussions and, essentially, no false-positives in normal patients,” said senior author Roland Lee, MD, professor of radiology and director of Neuroradiology, MRI and MEG at UC San Diego School of Medicine and VASDHS. “This makes it an ideal technique to monitor the effects of concussion treatments such as LIP-tES.”
The researchers found that the brains of all six participants displayed abnormal slow-waves in initial, baseline MEG scans. Following treatment using IASIS, MEG scans indicated measurably reduced abnormal slow-waves. The participants also reported a significant reduction in post-concussion scores.
“For the first time, we’ve been able to document with neuroimaging the effects of LIP-tES treatment on brain functioning in mild TBI,” said first author Ming-Xiong Huang, PhD, professor in the Department of Radiology at UC San Diego School of Medicine and a research scientist at VASDHS. “It’s a small study, which certainly must be expanded, but it suggests new potential for effectively speeding the healing process in mild traumatic brain injuries.”
Co-authors include: Ashley Robb Swan, Annemarie Angeles Quinto, Scott Matthews, Deborah L. Harrington, Sharon Nichols, Charles W. Huang, and Dewleen G. Baker, UC San Diego and VASDHS; Barry J. Bruder, IASIS Technologies, Los Angeles; and Corey C. Snook, Mind-Brain Training Institute, Mount Dora, FL.
Funding for this research came, in part, from the U.S. Department of Veterans Affairs (I01-CX000499, I01-RX001988, MHBA-010-14F, NURC-022-10F, NEUC-044-065).
Disclosure: Barry Bruder and Corey Snook are associated with IASIS Technologies. Their contribution to this work was limited to providing group training to researchers, technical support and technical information related to the IASIS system.
A pilot treatment study for mild traumatic brain injury: Neuroimaging changes detected by MEG after low-intensity pulse-based transcranial electrical stimulation
Background:
Mild traumatic brain injury (mTBI) is a leading cause of sustained impairments in military service members, Veterans, and civilians. However, few treatments are available for mTBI, partially because the mechanism of persistent mTBI deficits is not fully understood.
Methods:
We used magnetoencephalography (MEG) to investigate neuronal changes in individuals with mTBI following a passive neurofeedback-based treatment programme called IASIS. This programme involved applying low-intensity pulses using transcranial electrical stimulation (LIP-tES) with electroencephalography monitoring. Study participants included six individuals with mTBI and persistent post-concussive symptoms (PCS). MEG exams were performed at baseline and follow-up to evaluate the effect of IASIS on brain functioning.
Results:
At the baseline MEG exam, all participants had abnormal slow-waves. In the follow-up MEG exam, the participants showed significantly reduced abnormal slow-waves with an average reduction of 53.6 ± 24.6% in slow-wave total score. The participants also showed significant reduction of PCS scores after IASIS treatment, with an average reduction of 52.76 ± 26.4% in PCS total score.
Conclusions:
The present study demonstrates, for the first time, the neuroimaging-based documentation of the effect of LIP-tES treatment on brain functioning in mTBI. The mechanisms of LIP-tES treatment are discussed, with an emphasis on LIP-tES’s potentiation of the mTBI healing process.
Mild traumatic brain injury (mTBI) is a leading cause of sustained impairments in military service members, Veterans, and civilians. However, few treatments are available for mTBI, partially because the mechanism of persistent mTBI deficits is not fully understood.
Methods:
We used magnetoencephalography (MEG) to investigate neuronal changes in individuals with mTBI following a passive neurofeedback-based treatment programme called IASIS. This programme involved applying low-intensity pulses using transcranial electrical stimulation (LIP-tES) with electroencephalography monitoring. Study participants included six individuals with mTBI and persistent post-concussive symptoms (PCS). MEG exams were performed at baseline and follow-up to evaluate the effect of IASIS on brain functioning.
Results:
At the baseline MEG exam, all participants had abnormal slow-waves. In the follow-up MEG exam, the participants showed significantly reduced abnormal slow-waves with an average reduction of 53.6 ± 24.6% in slow-wave total score. The participants also showed significant reduction of PCS scores after IASIS treatment, with an average reduction of 52.76 ± 26.4% in PCS total score.
Conclusions:
The present study demonstrates, for the first time, the neuroimaging-based documentation of the effect of LIP-tES treatment on brain functioning in mTBI. The mechanisms of LIP-tES treatment are discussed, with an emphasis on LIP-tES’s potentiation of the mTBI healing process.
Passive Neurofeedback Treatments for Mild Traumatic Brain Injury: Neuroimaging Changes Detected by MEG after applying Low-Intensity Pulses using Transcranial Electrical Stimulation |
Ming-Xiong Huang,1,2* Ashley Robb Swan,1,2 Annemarie Angeles Quinto,1,2 Scott Matthews,3,4 Deborah L. Harrington,1,2 Sharon Nichols,5 Barry J. Bruder,6 Corey C. Snook,6 Charles W. Huang,7 Dewleen G. Baker,2,8,9 Roland R. Lee1,2
1Department of Radiology, University of California, San Diego, CA, USA
2Radiology, Research, and Psychiatry Services, VA San Diego Healthcare System, San Diego, CA, USA
3Department of Psychiatry, University of California, San Diego, CA, USA
4ASPIRE Center, VASDHS Residential Rehabilitation Treatment Program, San Diego, CA, USA
5Department of Neuroscience, University of California, San Diego, CA, USA
6IASIS Technologies, Inc. Los Angeles, CA, USA
7Department of Neuroscience, University of California, San Diego, CA, USA
8Department of Psychiatry, University of California, San Diego, CA, USA
9VA Center of Excellence for Stress and Mental Health, San Diego, CA, USA
*Correspondence: Ming-Xiong Huang, Ph.D., Professor of Radiology
Radiology Imaging Laboratory, University of California at San Diego, 3510 Dunhill Street, San Diego, CA 92121, USA. Tel: +1-858-534-1254, Fax: +1-858-534-6046, Email: [email protected]
1Department of Radiology, University of California, San Diego, CA, USA
2Radiology, Research, and Psychiatry Services, VA San Diego Healthcare System, San Diego, CA, USA
3Department of Psychiatry, University of California, San Diego, CA, USA
4ASPIRE Center, VASDHS Residential Rehabilitation Treatment Program, San Diego, CA, USA
5Department of Neuroscience, University of California, San Diego, CA, USA
6IASIS Technologies, Inc. Los Angeles, CA, USA
7Department of Neuroscience, University of California, San Diego, CA, USA
8Department of Psychiatry, University of California, San Diego, CA, USA
9VA Center of Excellence for Stress and Mental Health, San Diego, CA, USA
*Correspondence: Ming-Xiong Huang, Ph.D., Professor of Radiology
Radiology Imaging Laboratory, University of California at San Diego, 3510 Dunhill Street, San Diego, CA 92121, USA. Tel: +1-858-534-1254, Fax: +1-858-534-6046, Email: [email protected]
AbstractMild traumatic brain injury (mTBI) is a leading cause of sustained impairments in military service members, Veterans, and the general civilian population. However, few treatments are available for mTBI, partially because the mechanism of persistent mTBI deficits is not fully understood. The present study used magnetoencephalography (MEG) to investigate neuronal changes in the brain in individuals with mTBI in response to a passive neurofeedback-based treatment program called IASIS. This program involved applying low-intensity pulses using transcranial electrical stimulation (LIP-tES) with electroencephalography (EEG) monitoring. Study participants included 6 individuals with mTBI and persistent post-concussive symptoms (PCS). IASIS treatments were applied twice weekly for a period of 6 weeks. MEG exams were performed at baseline and follow-up to evaluate the effect of the IASIS treatment on brain functioning. We focused on changes in MEG source-imaging measures of abnormal slow-frequency activity and its relationship to changes in PCS. The results showed that at the baseline MEG exam, all participants had abnormal slow-wave signals. In the MEG exam following the IASIS treatment, the participants showed significantly reduced abnormal slow-waves in approximately the same brain areas that generated abnormal slow-waves at the baseline exam. Significant reductions in abnormal MEG slow-wave generation strongly correlated with significant reductions in PCS scores after the IASIS treatment. Altogether, the present study demonstrates, for the first time, neuroimaging-based documentation of the effect of LIP-tES treatment on brain functioning in individuals with mTBI.
Keywords: Magnetoencephalography, traumatic brain injury, slow-wave, Low Energy Neurofeedback System, Flexyx Neurotherapy System
IntroductionTraumatic brain injury (TBI) is a leading cause of sustained physical, cognitive, emotional, and behavioral deficits in the civilian population and military personnel. The majority of TBIs are in the “mild” range of severity. Mild TBI (mTBI) accounts for 75% of civilian TBIs from motor vehicle accidents, sports, falls, and assaults (Centers for Disease Control and Prevention and National Center for Injury Prevention and Control, 2003). In active-duty military personnel and combat Veterans, the majority (89%) of TBIs from blast injuries are also mild (MacGregor et al., 2011). However, the pathophysiology of mTBI is not completely understood and the long-term effects of mTBI are controversial. In the majority of individuals with mTBI, symptoms resolve within days post injury (Bigler, 2008). Yet, post-concussive symptoms (PCS) can persist for 3 months post injury or longer, indicating chronic sequelae. Estimates of the prevalence of persistent PCS vary widely. In a general civilian population, between 8% to 33% of mTBI patients show persistent long-term cognitive and/or behavioral impairments (Alexander, 1995; Binder, 1997, 1986; Bohnen et al., 1992; McCauley et al., 2013; Rimel et al., 1981; Rutherford, 1989). In Veterans with combat-related mTBI, at least three enduring PCS were reported in 7.5% to 40% of patients (Cooper et al., 2015; Morissette et al., 2011; Schneiderman et al., 2008; Terrio et al., 2009). It is unknown why similar acute mTBI events can lead to dramatic neurobehavioral decompensation with persistent PCS in some individuals, but not in others (Jeter et al., 2013). Optimal rehabilitation treatments for chronic PCS in mTBIs are also not understood, owing to insufficient information about the loci and mechanisms of injuries and the absence of neuroimaging-based assessments of treatment efficacy.
A promising class of treatments for mTBI is passive neurofeedback that applies low-intensity pulses using transcranial electrical stimulation (LIP-tES) with electroencephalography (EEG) monitoring. This class of EEG neurofeedback treatments use a common hardware design, but different software/protocol approaches; examples include Low Energy Neurofeedback System (LENS) (Ochs, 2006), Flexyx Neurotherapy System (FNS) (Ochs, 1997), and IASIS (the Greek word for healing or cure) (Snook, 2013). These treatments usually fall within the bio-energy domain of complementary and alternative medicine; they offset brain-wave activity by applying LIP-tES using the same EEG cables and electrodes that measure the brain waves (Nelson and Esty, 2015a). LENS and FNS have been used to treat individuals with TBI, including mTBI, showing positive effects on behavioral sequelae (Larsen et al., 2006; Nelson and Esty, 2015a, 2015b, 2012; Schoenberger et al., 2001). In addition, Larsen and colleagues reported that neurofeedback treatment significantly decreased EEG amplitude at the highest amplitude electrode site and at electrode Cz in a mixed population of individuals with TBI and other disorders (Larsen et al., 2006). It was not clear exactly why lower EEG amplitude was beneficial, though it was perceived that LIP-tES can offset the brain waves from its dominant frequency, presumably in an abnormal state (Larsen et al., 2006; Ochs, 2006). However, the underlying mechanism(s) of efficacy of LIP-tES treatment in TBI are not understood, nor have they been studied in animal models. No neuroimaging studies have assessed neuronal changes in the brain after LIP-tES treatment in individuals with TBI, and their relationship to improved behavioral outcomes.
In this regard, magnetoencephalography (MEG) is of keen interest because it is a non-invasive functional imaging technique that directly measures the neuronal current in gray matter (GM) with high temporal resolution (< 1 ms) and good spatial localization accuracy (2-3 mm at cortical level) (Leahy et al., 1998). MEG is highly sensitive to abnormal slow-wave signals (delta-band 1-4 Hz, extending to theta-band 5-7 Hz) in mTBI (M. Huang et al., 2016; Huang et al., 2014b, 2012, 2009, Lewine et al., 2007, 1999; Robb Swan et al., 2015). Neurophysiological studies in animals have established a solid connection between pathological delta-wave generation in GM and axonal injuries (Ball et al., 1977; Gloor et al., 1977) or neurochemical damage (e.g., cholinergic pathway injury) (Schaul et al., 1978) in white-matter (WM). Indeed, using diffusion tensor imaging (DTI) we found that abnormalities in underlying WM tracts in some mTBI individuals were related to abnormal MEG slow-waves (Huang et al., 2009). Using both region-of-interest (ROI) and voxel-wise approaches, we also showed that MEG slow-wave source imaging can detect abnormal slow-waves with ~85% sensitivity in patients with persistent PCS in chronic and sub-acute phases of mTBI (Huang et al., 2014b, 2012).
The present study used resting-state MEG to identify functional mechanisms associated with twice-weekly IASIS treatments for 6 weeks in individuals with chronic mTBI and persisting PCS. MEG source imaging changes in abnormal slow-waves were studied before and after IASIS treatments in mTBI participants. In addition, we examined whether changes in PCS after treatments were associated with changes in abnormal MEG slow-waves. Our main hypothesis was that IASIS treatment would be associated with significantly decrease abnormal MEG slow-waves and PCS in mTBI individuals relative to the pre-treatment baseline, and the MEG slow-wave changes would correlate with changes in PCS.
Keywords: Magnetoencephalography, traumatic brain injury, slow-wave, Low Energy Neurofeedback System, Flexyx Neurotherapy System
IntroductionTraumatic brain injury (TBI) is a leading cause of sustained physical, cognitive, emotional, and behavioral deficits in the civilian population and military personnel. The majority of TBIs are in the “mild” range of severity. Mild TBI (mTBI) accounts for 75% of civilian TBIs from motor vehicle accidents, sports, falls, and assaults (Centers for Disease Control and Prevention and National Center for Injury Prevention and Control, 2003). In active-duty military personnel and combat Veterans, the majority (89%) of TBIs from blast injuries are also mild (MacGregor et al., 2011). However, the pathophysiology of mTBI is not completely understood and the long-term effects of mTBI are controversial. In the majority of individuals with mTBI, symptoms resolve within days post injury (Bigler, 2008). Yet, post-concussive symptoms (PCS) can persist for 3 months post injury or longer, indicating chronic sequelae. Estimates of the prevalence of persistent PCS vary widely. In a general civilian population, between 8% to 33% of mTBI patients show persistent long-term cognitive and/or behavioral impairments (Alexander, 1995; Binder, 1997, 1986; Bohnen et al., 1992; McCauley et al., 2013; Rimel et al., 1981; Rutherford, 1989). In Veterans with combat-related mTBI, at least three enduring PCS were reported in 7.5% to 40% of patients (Cooper et al., 2015; Morissette et al., 2011; Schneiderman et al., 2008; Terrio et al., 2009). It is unknown why similar acute mTBI events can lead to dramatic neurobehavioral decompensation with persistent PCS in some individuals, but not in others (Jeter et al., 2013). Optimal rehabilitation treatments for chronic PCS in mTBIs are also not understood, owing to insufficient information about the loci and mechanisms of injuries and the absence of neuroimaging-based assessments of treatment efficacy.
A promising class of treatments for mTBI is passive neurofeedback that applies low-intensity pulses using transcranial electrical stimulation (LIP-tES) with electroencephalography (EEG) monitoring. This class of EEG neurofeedback treatments use a common hardware design, but different software/protocol approaches; examples include Low Energy Neurofeedback System (LENS) (Ochs, 2006), Flexyx Neurotherapy System (FNS) (Ochs, 1997), and IASIS (the Greek word for healing or cure) (Snook, 2013). These treatments usually fall within the bio-energy domain of complementary and alternative medicine; they offset brain-wave activity by applying LIP-tES using the same EEG cables and electrodes that measure the brain waves (Nelson and Esty, 2015a). LENS and FNS have been used to treat individuals with TBI, including mTBI, showing positive effects on behavioral sequelae (Larsen et al., 2006; Nelson and Esty, 2015a, 2015b, 2012; Schoenberger et al., 2001). In addition, Larsen and colleagues reported that neurofeedback treatment significantly decreased EEG amplitude at the highest amplitude electrode site and at electrode Cz in a mixed population of individuals with TBI and other disorders (Larsen et al., 2006). It was not clear exactly why lower EEG amplitude was beneficial, though it was perceived that LIP-tES can offset the brain waves from its dominant frequency, presumably in an abnormal state (Larsen et al., 2006; Ochs, 2006). However, the underlying mechanism(s) of efficacy of LIP-tES treatment in TBI are not understood, nor have they been studied in animal models. No neuroimaging studies have assessed neuronal changes in the brain after LIP-tES treatment in individuals with TBI, and their relationship to improved behavioral outcomes.
In this regard, magnetoencephalography (MEG) is of keen interest because it is a non-invasive functional imaging technique that directly measures the neuronal current in gray matter (GM) with high temporal resolution (< 1 ms) and good spatial localization accuracy (2-3 mm at cortical level) (Leahy et al., 1998). MEG is highly sensitive to abnormal slow-wave signals (delta-band 1-4 Hz, extending to theta-band 5-7 Hz) in mTBI (M. Huang et al., 2016; Huang et al., 2014b, 2012, 2009, Lewine et al., 2007, 1999; Robb Swan et al., 2015). Neurophysiological studies in animals have established a solid connection between pathological delta-wave generation in GM and axonal injuries (Ball et al., 1977; Gloor et al., 1977) or neurochemical damage (e.g., cholinergic pathway injury) (Schaul et al., 1978) in white-matter (WM). Indeed, using diffusion tensor imaging (DTI) we found that abnormalities in underlying WM tracts in some mTBI individuals were related to abnormal MEG slow-waves (Huang et al., 2009). Using both region-of-interest (ROI) and voxel-wise approaches, we also showed that MEG slow-wave source imaging can detect abnormal slow-waves with ~85% sensitivity in patients with persistent PCS in chronic and sub-acute phases of mTBI (Huang et al., 2014b, 2012).
The present study used resting-state MEG to identify functional mechanisms associated with twice-weekly IASIS treatments for 6 weeks in individuals with chronic mTBI and persisting PCS. MEG source imaging changes in abnormal slow-waves were studied before and after IASIS treatments in mTBI participants. In addition, we examined whether changes in PCS after treatments were associated with changes in abnormal MEG slow-waves. Our main hypothesis was that IASIS treatment would be associated with significantly decrease abnormal MEG slow-waves and PCS in mTBI individuals relative to the pre-treatment baseline, and the MEG slow-wave changes would correlate with changes in PCS.
Methods and Materials The study protocol was approved by institutional review boards of the University of California, San Diego. All participants gave written informed consent prior to study procedures. The informed consent followed the ethical guidelines of the Declarations of Helsinki (sixth revision, 2008).
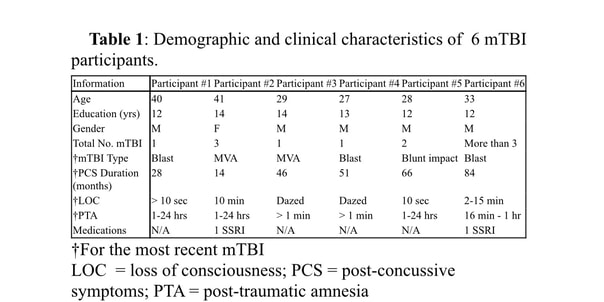
Research ParticipantsTable 1 describes demographic and clinical characteristics of the 6 study participants. All participants had a chronic mTBI with persistent PCS for an average of 48.2 (± 25.2) months between the most recent mTBI and the baseline interview (see below). Three participants had multiple mTBIs. For the most recent incident, causes of injury included blast (n=3), motor vehicle accident (MVA; n=2), and blunt trauma (n=1).
Participants were evaluated in a baseline interview to assess the nature of their injuries and persistent PCS. The diagnosis and classification of mTBI participants were based on standard VA and Department of Defense (DOD) diagnostic criteria (The Management of Concussion/mTBI Working Group, 2009). Inclusion criteria for mTBI participants included the following: 1) loss of consciousness (LOC) < 30 minutes or transient confusion, disorientation, or impaired consciousness immediately after the trauma; 2) post-traumatic amnesia (PTA) < 24 hours; and 3) initial Glasgow Coma Scale (GCS) (Teasdale and Jennett, 1974) between 13-15 (if available). Since the GCS assessment was often not available, individuals with missing GCS, but who met other inclusion criteria, were also enrolled. Table 1 lists the LOC and PTA information for the most recent mTBI, and the total number of TBIs for each participant.
Exclusion criteria for study participation included: 1) history of other neurological, developmental or psychiatric disorders (e.g., brain tumor, stroke, epilepsy, Alzheimer disease, or schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, or diagnosis of learning disability); 2) diagnosis of major depressive disorder (MDD) prior to the mTBI; 3) substance or alcohol use disorder according to DSM-V criteria within the three months prior to the study, based on a clinical interview; 4) history of metabolic or other diseases known to affect the central nervous system (see (Dikmen et al., 1995) for similar criteria); 5) extensive metal dental hardware (e.g., braces and large metal dentures; fillings were acceptable) or other metal objects in the head, neck, or face areas that could cause artifacts in the MEG data, not removable during pre-processing; and 6) suicidal ideation as evaluated using the Beck Depression Inventory (BDI-II), i.e., any participant reporting a score of “2” or “3” on the BDI –II: item 9 (suicidal thoughts or wishes), confirmed in follow-up risk assessment.
All participants were allowed to be on their currently prescribed medications, but to remain on the same medication regimen as best they could during the course of the IASIS treatment. As listed in Table 1, 2 participants were taking an anti-depressant SSRI; they remained on the SSRI and kept the same dosage throughout IASIS treatment. The rest of the participants were not on any medications. Past history of drug and alcohol use were elicited in detail in the screening interview. Additionally, participants were asked to refrain from drinking alcohol or using illicit drugs the night before the MEG scan.
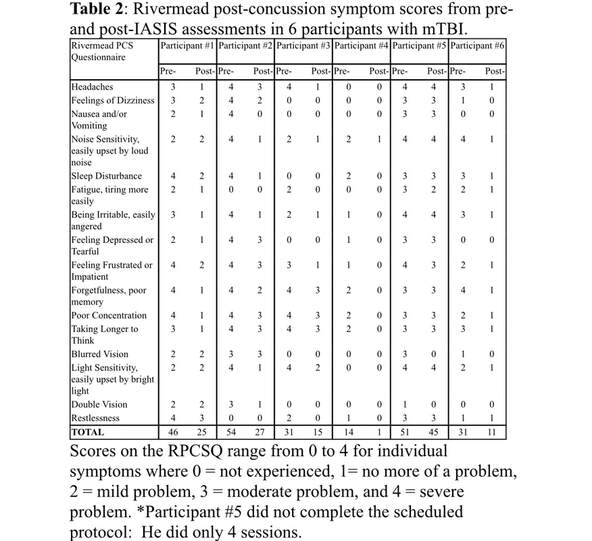
In the baseline interview, PCS in all mTBI participants were assessed using the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ) (King et al., 1995). This questionnaire contains 16 categories: 1) headaches; 2) feelings of dizziness; 3) nausea and/or vomiting; 4) noise sensitivity, easily upset by loud noise; 5) sleep disturbance; 6) fatigue, tiring more easily; 7) being irritable, easily angered; 8) feeling depressed or tearful; 9) feeling frustrated or impatient; 10) forgetfulness, poor memory; 11) poor concentration; 12) taking longer to think; 13) blurred vision; 14) light sensitivity, easily upset by bright light; 15) double vision; and 16) restlessness. The RPCSQ measures the extent to which a symptom is problematic, where 0 = not experienced, 1= no more of a problem, 2 = mild problem, 3 = moderate problem, and 4 = severe problem. Only participants with persistent symptoms in at least three of the above categories during the baseline assessment were recruited into the study. After the IASIS treatment, the same RPCSQ was used to assess the post-treatment PCS in the mTBI participants during a follow-up interview. One of the main study outcomes was the change in RPCSQ scores from pre- to post-IASIS treatment.
IASIS LIP-tES Treatment Procedure:IASIS (Micro Current Neurofeedback) is typically a 10-week (two 30-minute sessions per week) passive neurofeedback intervention with EEG monitoring. Due to study participant availability and resource constraints, we utilized a 6-week, 2 session per week intervention program. The IASIS device uses 5 EEG electrodes. The EEG interface device is the J&J Engineering I-330 C2, provided specifically for, and labeled for, IASIS, in accordance with specifications by Mind-Brain Training Institute (www.mind-braintraining.com). The software is IASIS 5.0, and it is supported by Physiolab 2007 supplied by J&J Engineering. The EEG sample frequency is 256 samples per second on each of 2 EEG acquisition channels.
The feedback LIP-tES is delivered via the 4 EEG leads (A+, A-, B+, B-), with respect to the Common Neck Reference (isolated). During each session, 2 electrodes (A- and B-) are attached to the participant’s left and right mastoids, while the remaining two electrodes (A+ and B+) are moved to various locations on the scalp. All four (A+, A-, B+, B-) electrodes are involved in applying weak electric current pulses back to the brain (the feedback process). The feedback signal consists of very narrow pulses, about 120 ns in duration, and 150 mV in amplitude for all 3 Schedules (see below). The pulses are both positive-going and negative-going with respect to Common (tiny currents flow in both directions). The repetition rate of the feedback pulse train is determined dynamically by adding the dominant brainwave (EEG) frequency, selected from the range of 2 Hz - 12.5 Hz, to the frequency specified in each time interval of the 3 pre-programmed Schedules (see below) used in the study. The dominate frequency was acquired from the difference EEG signal between the A+ and A- electrodes using a trailing window Fast-Fourier Transform (FFT). For example, in an mTBI participant, the IASIS first picks up the dominant brain rhythms (e.g., 3.5 Hz). Next, the feedback electric pulses are sent back to the brain, with a repetition rate at various incremental frequencies added to the dominant one, e.g., first adding 2 Hz to the 3.5 Hz, and then adding 3.3 Hz, etc., changing each 5 seconds throughout the 25-second-long protocol. These feedback pulses seem to result in the disruption of the dominant brain rhythms (Ochs, 2006).
Usually, the A+ and B+ electrodes are moved to various places during each treatment session. However, this step may disrupt the treatment and also draw unnecessary attention from the participant to specific electrode sites. In the present study, we prepped and pre-placed a set of electrodes on the scalp of the participant following the 10-20 EEG configuration. These 10-20 sites were the potential sites for the A+ and B+ electrodes. During the treatment sessions, the electrode inputs to the EEG interface device for the A+ and B+ electrodes (selected from the pre-placed 10-20 configuration) were switched, without the participant’s knowledge, so that the participant could not tell which sites were activated. Out of the standard 10-20 electrode sites, electrode pairs activated were: F3/F4, C3/C4, P3/P4, O1/O2, T5/T6, Fz/Pz, FPz/Cz, FP1/FP2, F9/F10, F9/FC3. Each Schedule takes between 22-25 seconds per site and each Schedule was delivered twice per electrode pair.
For the purpose of standardizing the research protocol, we focused on three different Schedules: Genesis, Balanced Energy, and Activation (see Appendix I for details of each Schedule). Genesis Schedule was performed during the first two visits. Balanced Energy Schedule was performed for the 3rd and 4th visit. This Schedule provides a set of varied offset frequencies that change at 2-second intervals. Activation Schedule was performed for the eight remaining visits. The EEG interface device (J&J I-330 C2) that we employ puts out a "background" (unrelated to participant EEG) signal, with narrow pulses, in the approximately 1 KHz repetition rate range. The rate is modulated upward, creating a kind of "chirp", at each repaint of the windows frame.
MEG Data Acquisition and Signal Pre-processing to Remove ArtifactsResting-state MEG data were collected using the VectorViewä whole-head MEG system (Elekta-Neuromag, Helsinki, Finland) with 306 MEG channels. Participants were seated in upright position inside a multi-layer magnetically-shielded room (IMEDCO-AG) (Cohen et al., 2002) at the UCSD MEG Center. For each participant, two 5-minute sessions with eyes closed were acquired. The participants were instructed to empty their minds and to avoid moving their eyes. Data were sampled at 1000 Hz and were run through a high-pass filter with a 0.1 Hz cut-off, and a low-pass filter with a 330 Hz cut-off. Eye blinks and eye movements were monitored using two pairs of bipolar electrodes with one pair placed above and below the left eye, and the other pair placed on the two temples. Heart signals were monitored with another pair of bipolar electrodes. Precautions were taken to ensure head stability: foam wedges were inserted between the participant’s head and the inside of the unit, and a Velcro strap was placed under the participant’s chin and anchored in superior and posterior axes. Head movement across different sessions was about 2-3 mm on average.
To help ensure that participants were alert during the MEG recordings, prior to each of the study sessions, they completed a questionnaire about the number of hours they slept the previous night, how rested they felt, and if there was any reason that they might not be attentive and perform to the best of their abilities (due to headache, pain, etc.). Participants were scheduled early in the day to avoid fatigue from performing daily activities. The amount of alpha band oscillations, which is consistently associated with tonic alertness, was also monitored online to gauge the cognitive state of participants. Participants were viewed on a camera, which also allowed for monitoring alertness of each participant.
MEG eyes-closed data were first run through MaxFilter, also known as signal space separation (Song et al., 2008; Taulu et al., 2004a, 2004b), to remove external interferences (e.g., magnetic artifacts due to metal objects, strong cardiac signals, environment noises, etc.). Next, residual artifacts near the sensor array due to eye movements and residual cardiac signals were removed using Independent Component Analysis using Fast-ICA (http://research.ics.aalto.fi/ica/fastica/) (Hyvarinen, 1999; Hyvarinen and Oja, 2000). The waveforms associated with top independent components (ICs) were examined by an experienced MEG data analyst, along with ECG and EOG signals. ICs associated with eye blinks, eye movements, heartbeats, and other artifacts were removed.
Structural MRI, MEG-MRI Registration, BEM Forward Calculation Structural MRI of the participant’s head was collected using a General Electric 1.5T Excite MRI scanner. The acquisition contains a standard high-resolution anatomical volume with a resolution of 0.94´0.94´1.2 mm3 using a T1-weighted 3D-IR-FSPGR pulse sequence. Scanner-related imaging distortions were corrected using a gradient non-linearity correction approach (Jovicich et al., 2006). To co-register the MEG with MRI coordinate systems, three anatomical landmarks (i.e., left and right pre-auricular points, and nasion) were measured for each participant using the Probe Position Identification system (Polhemus, USA). By identifying the same three points on the participant's MR images using MRILAB (Elekta/Neuromag), a transformation matrix involving both rotation and translation between the MEG and MR coordinate systems was generated. To increase the reliability of the MEG-MR co-registration, at least 150 points on the scalp were digitized with the Polhemus system, in addition to the three landmarks, and those points were co-registered onto the scalp surface of the MR images. The T1-weighted images were also used to extract the brain volume and innermost skull surface (SEGLAB software developed by Elekta/Neuromag). Realistic Boundary Element Method (BEM) head model was used for MEG forward calculation.(Huang et al., 2007; Mosher et al., 1999) The BEM mesh was constructed by tessellating the inner skull surface from the T1-weighted MRI into ~6000 triangular elements with ~5 mm size. A cubic source grid with 5 mm size covering cortical and subcortical GM areas based on FCONN brain parcellation (Shen et al., 2013) was created. Such a source grid was used for calculating the MEG gain (i.e., lead-field) matrix, which leads to a grid with ~10,000 nodes covering the whole brain. Then, the source grid was combined with the BEM mesh in the MRI coordinate for the BEM forward calculation.
Other conventional MRI sequences typical for identifying structural lesions in TBI participants were also performed: 1) Axial T2*-weighted; 2) Axial fast spin-echo T2-weighted; and 3) Axial FLAIR. The conventional MRIs were carefully reviewed by a Board-certified neuroradiologist (R.R. Lee); no visible lesions were found on the MRI of any participant.
MEG Source Magnitude Imaging using Fast-VESTAL In both 5-minute rs-MEG data sessions with eyes closed, sensor-waveforms were run through a band-pass filter for 1 - 4 Hz (delta band). The data set was then divided into 2.5-second duration epochs, and sensor waveform covariance matrices were calculated for each epoch. A total sensor-waveform covariance matrix for the entire 10-minute recording was calculated by concatenating the covariance matrices from individual epochs. Using the total covariance matrix, voxel-wise MEG slow-wave source magnitude images that cover the whole brain were obtained for each participant following the Fast-VESTAL procedure (Huang et al., 2014a). An Objective Pre-whitening Method was applied to remove correlated environmental noise and objectively select the dominant eigen-modes of sensor-waveform covariance matrix (Huang et al., 2014a).
The Fast-VESTAL technique consists of two steps. First, spatio-temporal L1-minimum-norm MEG source images were obtained for the dominant spatial (i.e., eigen-) modes of sensor-waveform covariance matrix. Next, accurate source time-courses were obtained using an inverse operator constructed from the spatial source images of Step 1. This approach has been successfully used to obtain comprehensive MEG source-magnitude images covering the entire brain for different frequency bands of resting-state brain rhythms (Huang et al., 2014a). The second-order cone programming (SOCP) approach in the minimum L1-norm solver of the SeDuMi software (http://sedumi.ie.lehigh.edu/) was used in the present study. SOCP corrects orientation bias in a one-step approach (Haufe et al., 2011; Ou et al., 2008). The technical details of Fast-VESTAL using the SOCP formulation is in the appendix of our recent paper (C. W. Huang et al., 2016).
Characterizing Abnormal MEG Slow-wave Source Imaging in Individual mTBI Participants The procedure for detecting abnormal MEG slow-wave in single subjects using a voxel-wise approach is detailed in our previous study (Huang et al., 2014b). In the present study, MEG slow-waves in single subjects were evaluated against our voxel-wise normative database that contains 96 healthy individuals between the ages of 18 to 55. The normative database is in MNI-152 atlas coordinates for the MEG source magnitude (spatially smoothed and logarithm transformed). After spatial smoothing and logarithm transformation, the MEG source magnitude images from each mTBI participant were registered to the MNI-152 coordinates, and then converted into Z-score maps using the voxel-wise normative database (Huang et al., 2014b). The abnormal MEG slow-wave generation from each mTBI participant was characterized by the voxels in the Z-score maps with statistical significance (q-value <0.01) after controlling the family-wise error due to multiple comparisons using false discovery rate approach (Benjamini and Hochberg, 1995). We also calculated total abnormal MEG Z-scores by summing up the Z-score from all voxels that showed statistically significant slow-wave generation. The focus of our analyses were on the pre- and post-IASIS change in the abnormal MEG slow-wave generation for both voxel-wise Z-score maps and the total abnormal MEG Z-scores.
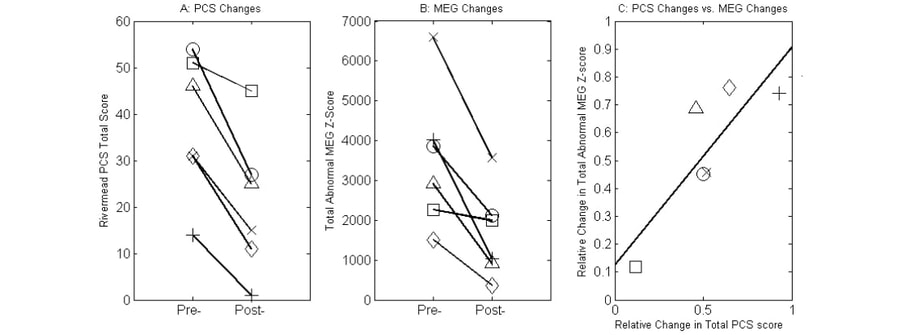
ResultsPre- and Post-IASIS Changes in PCS ScoresA key element of the present study was to study clinical symptom changes after IASIS treatment. We found significant reductions of clinical symptoms in the 6 mTBI individuals who participated in the IASIS treatment. Table 2 lists the RPCSQ scores from the pre- and post-IASIS assessments in each participant. PCS total scores across 16 categories were markedly reduced after the IASIS treatment in all participants (Table 2, bottom row; Figure 1A). The observed reduction in PCS total scores between the pre- and post-IASIS assessments was statistically significant (paired group t-test, t = 5.80, p < 0.01, Cohen's d = 2.37)
Pre- and Post-IASIS Changes in Total Abnormal Z-scores from MEG Slow-wave ImagingFig. 1B shows a striking reduction in the total abnormal Z-scores that measured the abnormal MEG slow-wave generation between the pre- and post-IASIS MEG exams. The change in total abnormal MEG Z-scores was also statistically significant (paired group t-test, t = 4.28, p < 0.01, Cohen's d = 1.75)
Next, we correlated the change in MEG slow-waves due to treatment with change in the total PCS scores. In this analysis, we examined two measures: 1) the absolute change of both total abnormal MEG Z-scores and the PCS scores, i.e., pre - post; and 2) the relative change for both total abnormal Z-scores from MEG slow-wave imaging and the PCS scores calculated according to the following formula: (pre - post) / pre. The results showed no significant correlation between absolute change of total abnormal MEG Z-scores and the PCS scores (r = 0.23, p = 0.65). However, relative total abnormal MEG Z-score change significantly correlated with relative total PCS score change. Fig. 1C showed a significant positive correlation between the relative total abnormal MEG Z-score change and relative total PCS score change (r = 0.84, p < 0.05).
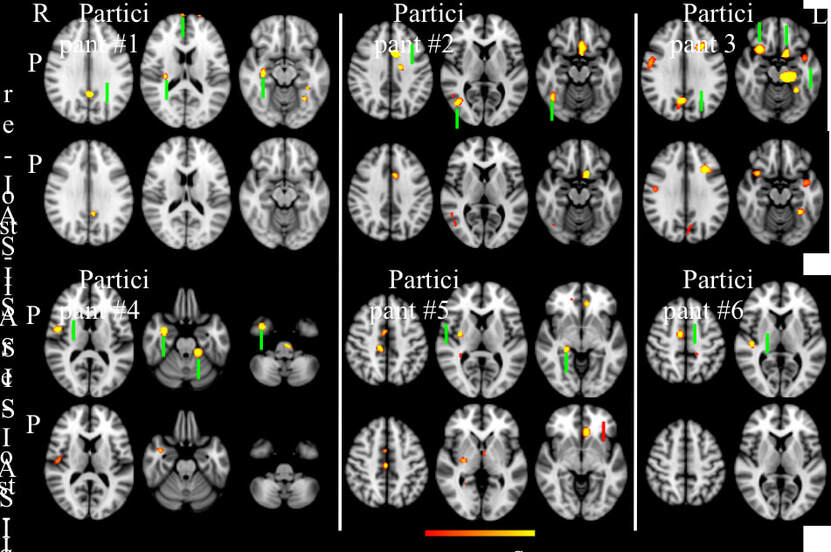
Pre- and Post-IASIS Changes in both PCS and MEG Slow-wave Imaging for Individual ParticipantsIn this subsection, detailed information is provided for each participant about pre- and post-IASIS changes related to PCS and abnormal voxel-wise MEG slow-waves. Fig. 2 shows the voxel-wise MEG findings for assessing the effect of IASIS treatment on brain functioning in all 6 mTBI participants. Representative axial slices with major abnormal slow-waves (q < 0.01, FDR correction) are plotted in Fig. 2.
Participant #1: During his tenure in the Marine Corps, Participant #1 experienced an mTBI due to a mortar blast, which caused LOC for over 10 seconds and post-traumatic amnesia for less than 24 hours. Upon entering the study, he reported multiple PCS symptoms persisting since the mortar blast, most notably headache, sleep disturbances, poor memory / poor concentration, and feelings of frustration (Table 2). On the RPCSQ scale, Participant #1’s most notable symptoms were ranked as moderate to severe.
During and following the IASIS treatment, Participant #1 reported that his symptoms greatly abated, going from severe to no problem or mild. His overall RPCSQ score went from 46 to 25, a reduction of 45.7%. The participant also mentioned that he had completely discontinued his use of nicotine after the IASIS treatments, which he claimed was a beneficial result of the treatments. To appreciate the longer lasting effects of the treatment, the participant stated that 6 months after IASIS that the treatment effects had lasted and that he still did not use nicotine.
Compared with pre-IASIS MEG, Participant #1's post-IASIS MEG showed marked reduction of 68.6% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed markedly reduced abnormal slow-waves from frontal pole, posterior cingulate cortex (PCC), right insula, and right hippocampus (Fig. 2). The MEG findings were compatible with reduced PCS for headaches (posterior insular), memory function (related to hippocampus), and feelings of frustration or impatience (frontal pole).
Participant #2: Participant #2 was involved in a multi-vehicle accident that resulted in a loss of consciousness for approximately 10 minutes, which led to mTBI with widespread moderate to severe symptoms. Symptoms included headaches, photophobia, stuttering, overstimulation, anxiety, memory loss, sleep disturbances, and depression. For example, during the initial interview of the study, her photophobia was so severe that we had to turn off all lights in the office to avoid triggering a severe headache.
Halfway through the IASIS sessions, Participant #2 reported a reduction in stuttering, anxiety, headaches, and less visual and auditory overstimulation (particularly reduction of photophobia). She was able go outside and participate in a yard-sale for her church during a sunny afternoon without any headache. After the completion of all IASIS treatments, she reported an even greater reduction of symptoms (i.e., by 50.0%). Her overall score for RPCSQ went from 54 to 27.
In Participant #2, the total abnormal MEG Z-score showed marked reduction of 45.1% in the post-IASIS versus pre-IASIS MEG exams (Figs. 1B and 1C). Specifically, markedly reduced MEG slow-waves were found in anterior cingulate cortex (ACC), right lateral occipital cortex, and right occipital fusiform gyrus (Fig. 2). The MEG findings were also compatible with reduced PCS for headaches (ACC) and photophobia (occipital cortex and fusiform gyrus).
Participant #3: Participant #3 was involved in a driver’s side car accident. The impact caused his head to hit and dent the roof of his car. Prior to the IASIS treatment, he noted severe problems with headaches, forgetfulness / poor memory, poor concentration, taking longer to think, and light sensitivity. He further noted mild problems with noise sensitivity, and irritability, and moderate problems with frustration.
After IASIS, his symptoms drastically reduced from an initial RPCSQ total score of 31 to 15, a reduction of 51.6%. Light sensitivity was reported as only mild whereas headaches, noise sensitivity, irritability, and frustration dropped to insignificant, i.e. no more of a problem. Importantly, severe symptoms became more moderate after treatment, including forgetfulness / poor memory, poor concentration, and taking longer to think.
Compared with the pre-IASIS exam, Participant #3’s post-IASIS MEG exam showed marked reduction of 45.8% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed markedly reduced abnormal slow-waves in PCC, bilateral OFC, and left hippocampus (Fig. 2). The MEG findings are compatible with reduced PCS for memory problems (hippocampus) and headaches (PCC).
Participant #4: While in the Army, Participant #4 reported experiencing an IED blast in which he felt a shock-wave while riding in an Mine-Resistant Ambush Protected vehicle. He was left dazed for a few seconds. Soon after, he had PCS which continued through the first MEG session. Before the IASIS sessions, the participant noted multiple symptoms including noise sensitivity, sleep disturbances, forgetfulness, poor concentration, and taking longer to think. All symptoms were in the mild range in the RPCSQ, with an overall score of 14.
After 3 visits and throughout the remaining IASIS sessions, Participant #4 reported that his quality of sleep had improved, leaving him well rested with a positive change in attitude. Upon finishing all IASIS sessions, this participant recorded an overall score of 1 on the RPCSQ, a reduction of 92.9% in total RPCSQ score. He noted that noise sensitivity was no more of a problem for him. All other symptoms were listed as absent, which meant that Participant #4 was essentially symptom-free.
The pre- and post-IASIS MEG exams show that Participant #4 had a marked reduction of 74.2% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, marked reductions of abnormal slow-waves were found in the right inferior-lateral parietal area / superior temporal gyrus, right hippocampus and amygdala, right inferior temporal pole, and left cerebellum (Fig. 2). The MEG findings were compatible with reduced PCS for noise sensitivity (superior temporal gyrus) and forgetfulness (hippocampus and inferior temporal area).
Participant #5: During his years in the Marine Corp, Participant #5 experienced blunt head trauma while furniture that was being moved struck him in the head. He experienced another blunt head trauma three months prior to starting IASIS when a chair that he was sitting in broke and he hit the back of his head on another chair, intensifying his previous symptoms. Before starting IASIS, Participant #5 listed severe symptoms on the RPCSQ for headaches, noise sensitivity, irritability, frustration, and light sensitivity. Multiple other symptoms such as feelings of dizziness, nausea, sleep disturbance, fatigue, feeling depressed, forgetfulness, having poor concentration, taking longer to think, having blurred vision, and restlessness were scored as moderate, with a total RPCSQ score of 51.
Participant #5 only finished 4 out of the 12 required IASIS treatment sessions. From the beginning, he missed or rescheduled multiple sessions. Because it appeared likely that he might not (actually he did not) finish his IASIS treatment, an MEG exam was performed following his 4th visit, and that MEG was used for this paper. After his 4th IASIS visit, there was a reduction of symptoms but not as remarkable as that of individuals who completed all sessions. His RPSCQ total score changed from 51 in pre-IASIS exam to 45 after the 4th session, with only a marginal reduction of 11.8%. On the RPSCQ, he still noted headaches, irritability, and light sensitivity as severe problems. Similar to pre-IASIS sessions, symptoms of dizziness, sleep disturbance, feeling depressed, forgetfulness, having poor concentration and taking longer to think remained as moderate problems. There were some reductions with noted symptoms of noise sensitivity and feelings of frustration that were reduced from severe to moderate. Experiencing fatigue and restlessness also were reduced from moderate to mild.
The pre-IASIS MEG exams show that Participant #5 had abnormal slow-wave generation from right ACC and PCC, right striatum / insular cortex, right parahippocampus, and left ventro-medial prefrontal cortex (vmPFC) (Fig. 2). In his MEG exam following the 4th IASIS treatment visit, his total abnormal MEG Z-score only showed only a marginal reduction of 12.0% (Figs. 1B and 1C). Specifically, his abnormal slow-wave generation from the right ACC and PCC remained essentially the same. Reduced slow-waves were observed from his right striatum / insular cortex and his right parahippocampus, but increased slow-wave generation was found from his left vmPFC (Fig. 2). The MEG findings were compatible with his persistent PCS at his 4th visit for headache (ACC and PCC) and irritability (vmPFC).
Participant #6: Participant #6, an Army Veteran, experienced an IED while riding in a humvee. He noted being dazed for a few seconds. Before starting IASIS, Participant #6 had a variety of symptoms listed on the RPCSQ with a total score of 31 initially from symptoms ranging as severe, moderate, and mild. The symptoms listed as severe included noise sensitivity and poor concentration; more moderate symptoms included headaches, sleep disturbance, irritability, and taking longer to think, and mild symptoms included fatigue, feeling frustrated, having poor concentration, and light sensitivity.
Throughout the IASIS sessions, Participant #6 noted improvement with sleep quality, accompanied by feeling more energetic. By the end of the sessions, the symptoms listed were all scored as no more of a problem. The total RPCSQ score reduced to 11, a marked reduction of 64.5%.
The pre- and post-IASIS MEG exams show that Participant #6 had a marked reduction of 76.1% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed marked reduction of abnormal slow-wave generation from the right auditory cortex in superior temporal gyrus as well as right supplementary motor area (SMA) and ACC (Fig. 2). The MEG findings were compatible with reduced PCS for noise sensitivity (auditory cortex in superior temporal gyrus), as well as headaches and poor concentration (SMA and ACC).
Research ParticipantsTable 1 describes demographic and clinical characteristics of the 6 study participants. All participants had a chronic mTBI with persistent PCS for an average of 48.2 (± 25.2) months between the most recent mTBI and the baseline interview (see below). Three participants had multiple mTBIs. For the most recent incident, causes of injury included blast (n=3), motor vehicle accident (MVA; n=2), and blunt trauma (n=1).
Participants were evaluated in a baseline interview to assess the nature of their injuries and persistent PCS. The diagnosis and classification of mTBI participants were based on standard VA and Department of Defense (DOD) diagnostic criteria (The Management of Concussion/mTBI Working Group, 2009). Inclusion criteria for mTBI participants included the following: 1) loss of consciousness (LOC) < 30 minutes or transient confusion, disorientation, or impaired consciousness immediately after the trauma; 2) post-traumatic amnesia (PTA) < 24 hours; and 3) initial Glasgow Coma Scale (GCS) (Teasdale and Jennett, 1974) between 13-15 (if available). Since the GCS assessment was often not available, individuals with missing GCS, but who met other inclusion criteria, were also enrolled. Table 1 lists the LOC and PTA information for the most recent mTBI, and the total number of TBIs for each participant.
Exclusion criteria for study participation included: 1) history of other neurological, developmental or psychiatric disorders (e.g., brain tumor, stroke, epilepsy, Alzheimer disease, or schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, or diagnosis of learning disability); 2) diagnosis of major depressive disorder (MDD) prior to the mTBI; 3) substance or alcohol use disorder according to DSM-V criteria within the three months prior to the study, based on a clinical interview; 4) history of metabolic or other diseases known to affect the central nervous system (see (Dikmen et al., 1995) for similar criteria); 5) extensive metal dental hardware (e.g., braces and large metal dentures; fillings were acceptable) or other metal objects in the head, neck, or face areas that could cause artifacts in the MEG data, not removable during pre-processing; and 6) suicidal ideation as evaluated using the Beck Depression Inventory (BDI-II), i.e., any participant reporting a score of “2” or “3” on the BDI –II: item 9 (suicidal thoughts or wishes), confirmed in follow-up risk assessment.
All participants were allowed to be on their currently prescribed medications, but to remain on the same medication regimen as best they could during the course of the IASIS treatment. As listed in Table 1, 2 participants were taking an anti-depressant SSRI; they remained on the SSRI and kept the same dosage throughout IASIS treatment. The rest of the participants were not on any medications. Past history of drug and alcohol use were elicited in detail in the screening interview. Additionally, participants were asked to refrain from drinking alcohol or using illicit drugs the night before the MEG scan.
In the baseline interview, PCS in all mTBI participants were assessed using the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ) (King et al., 1995). This questionnaire contains 16 categories: 1) headaches; 2) feelings of dizziness; 3) nausea and/or vomiting; 4) noise sensitivity, easily upset by loud noise; 5) sleep disturbance; 6) fatigue, tiring more easily; 7) being irritable, easily angered; 8) feeling depressed or tearful; 9) feeling frustrated or impatient; 10) forgetfulness, poor memory; 11) poor concentration; 12) taking longer to think; 13) blurred vision; 14) light sensitivity, easily upset by bright light; 15) double vision; and 16) restlessness. The RPCSQ measures the extent to which a symptom is problematic, where 0 = not experienced, 1= no more of a problem, 2 = mild problem, 3 = moderate problem, and 4 = severe problem. Only participants with persistent symptoms in at least three of the above categories during the baseline assessment were recruited into the study. After the IASIS treatment, the same RPCSQ was used to assess the post-treatment PCS in the mTBI participants during a follow-up interview. One of the main study outcomes was the change in RPCSQ scores from pre- to post-IASIS treatment.
IASIS LIP-tES Treatment Procedure:IASIS (Micro Current Neurofeedback) is typically a 10-week (two 30-minute sessions per week) passive neurofeedback intervention with EEG monitoring. Due to study participant availability and resource constraints, we utilized a 6-week, 2 session per week intervention program. The IASIS device uses 5 EEG electrodes. The EEG interface device is the J&J Engineering I-330 C2, provided specifically for, and labeled for, IASIS, in accordance with specifications by Mind-Brain Training Institute (www.mind-braintraining.com). The software is IASIS 5.0, and it is supported by Physiolab 2007 supplied by J&J Engineering. The EEG sample frequency is 256 samples per second on each of 2 EEG acquisition channels.
The feedback LIP-tES is delivered via the 4 EEG leads (A+, A-, B+, B-), with respect to the Common Neck Reference (isolated). During each session, 2 electrodes (A- and B-) are attached to the participant’s left and right mastoids, while the remaining two electrodes (A+ and B+) are moved to various locations on the scalp. All four (A+, A-, B+, B-) electrodes are involved in applying weak electric current pulses back to the brain (the feedback process). The feedback signal consists of very narrow pulses, about 120 ns in duration, and 150 mV in amplitude for all 3 Schedules (see below). The pulses are both positive-going and negative-going with respect to Common (tiny currents flow in both directions). The repetition rate of the feedback pulse train is determined dynamically by adding the dominant brainwave (EEG) frequency, selected from the range of 2 Hz - 12.5 Hz, to the frequency specified in each time interval of the 3 pre-programmed Schedules (see below) used in the study. The dominate frequency was acquired from the difference EEG signal between the A+ and A- electrodes using a trailing window Fast-Fourier Transform (FFT). For example, in an mTBI participant, the IASIS first picks up the dominant brain rhythms (e.g., 3.5 Hz). Next, the feedback electric pulses are sent back to the brain, with a repetition rate at various incremental frequencies added to the dominant one, e.g., first adding 2 Hz to the 3.5 Hz, and then adding 3.3 Hz, etc., changing each 5 seconds throughout the 25-second-long protocol. These feedback pulses seem to result in the disruption of the dominant brain rhythms (Ochs, 2006).
Usually, the A+ and B+ electrodes are moved to various places during each treatment session. However, this step may disrupt the treatment and also draw unnecessary attention from the participant to specific electrode sites. In the present study, we prepped and pre-placed a set of electrodes on the scalp of the participant following the 10-20 EEG configuration. These 10-20 sites were the potential sites for the A+ and B+ electrodes. During the treatment sessions, the electrode inputs to the EEG interface device for the A+ and B+ electrodes (selected from the pre-placed 10-20 configuration) were switched, without the participant’s knowledge, so that the participant could not tell which sites were activated. Out of the standard 10-20 electrode sites, electrode pairs activated were: F3/F4, C3/C4, P3/P4, O1/O2, T5/T6, Fz/Pz, FPz/Cz, FP1/FP2, F9/F10, F9/FC3. Each Schedule takes between 22-25 seconds per site and each Schedule was delivered twice per electrode pair.
For the purpose of standardizing the research protocol, we focused on three different Schedules: Genesis, Balanced Energy, and Activation (see Appendix I for details of each Schedule). Genesis Schedule was performed during the first two visits. Balanced Energy Schedule was performed for the 3rd and 4th visit. This Schedule provides a set of varied offset frequencies that change at 2-second intervals. Activation Schedule was performed for the eight remaining visits. The EEG interface device (J&J I-330 C2) that we employ puts out a "background" (unrelated to participant EEG) signal, with narrow pulses, in the approximately 1 KHz repetition rate range. The rate is modulated upward, creating a kind of "chirp", at each repaint of the windows frame.
MEG Data Acquisition and Signal Pre-processing to Remove ArtifactsResting-state MEG data were collected using the VectorViewä whole-head MEG system (Elekta-Neuromag, Helsinki, Finland) with 306 MEG channels. Participants were seated in upright position inside a multi-layer magnetically-shielded room (IMEDCO-AG) (Cohen et al., 2002) at the UCSD MEG Center. For each participant, two 5-minute sessions with eyes closed were acquired. The participants were instructed to empty their minds and to avoid moving their eyes. Data were sampled at 1000 Hz and were run through a high-pass filter with a 0.1 Hz cut-off, and a low-pass filter with a 330 Hz cut-off. Eye blinks and eye movements were monitored using two pairs of bipolar electrodes with one pair placed above and below the left eye, and the other pair placed on the two temples. Heart signals were monitored with another pair of bipolar electrodes. Precautions were taken to ensure head stability: foam wedges were inserted between the participant’s head and the inside of the unit, and a Velcro strap was placed under the participant’s chin and anchored in superior and posterior axes. Head movement across different sessions was about 2-3 mm on average.
To help ensure that participants were alert during the MEG recordings, prior to each of the study sessions, they completed a questionnaire about the number of hours they slept the previous night, how rested they felt, and if there was any reason that they might not be attentive and perform to the best of their abilities (due to headache, pain, etc.). Participants were scheduled early in the day to avoid fatigue from performing daily activities. The amount of alpha band oscillations, which is consistently associated with tonic alertness, was also monitored online to gauge the cognitive state of participants. Participants were viewed on a camera, which also allowed for monitoring alertness of each participant.
MEG eyes-closed data were first run through MaxFilter, also known as signal space separation (Song et al., 2008; Taulu et al., 2004a, 2004b), to remove external interferences (e.g., magnetic artifacts due to metal objects, strong cardiac signals, environment noises, etc.). Next, residual artifacts near the sensor array due to eye movements and residual cardiac signals were removed using Independent Component Analysis using Fast-ICA (http://research.ics.aalto.fi/ica/fastica/) (Hyvarinen, 1999; Hyvarinen and Oja, 2000). The waveforms associated with top independent components (ICs) were examined by an experienced MEG data analyst, along with ECG and EOG signals. ICs associated with eye blinks, eye movements, heartbeats, and other artifacts were removed.
Structural MRI, MEG-MRI Registration, BEM Forward Calculation Structural MRI of the participant’s head was collected using a General Electric 1.5T Excite MRI scanner. The acquisition contains a standard high-resolution anatomical volume with a resolution of 0.94´0.94´1.2 mm3 using a T1-weighted 3D-IR-FSPGR pulse sequence. Scanner-related imaging distortions were corrected using a gradient non-linearity correction approach (Jovicich et al., 2006). To co-register the MEG with MRI coordinate systems, three anatomical landmarks (i.e., left and right pre-auricular points, and nasion) were measured for each participant using the Probe Position Identification system (Polhemus, USA). By identifying the same three points on the participant's MR images using MRILAB (Elekta/Neuromag), a transformation matrix involving both rotation and translation between the MEG and MR coordinate systems was generated. To increase the reliability of the MEG-MR co-registration, at least 150 points on the scalp were digitized with the Polhemus system, in addition to the three landmarks, and those points were co-registered onto the scalp surface of the MR images. The T1-weighted images were also used to extract the brain volume and innermost skull surface (SEGLAB software developed by Elekta/Neuromag). Realistic Boundary Element Method (BEM) head model was used for MEG forward calculation.(Huang et al., 2007; Mosher et al., 1999) The BEM mesh was constructed by tessellating the inner skull surface from the T1-weighted MRI into ~6000 triangular elements with ~5 mm size. A cubic source grid with 5 mm size covering cortical and subcortical GM areas based on FCONN brain parcellation (Shen et al., 2013) was created. Such a source grid was used for calculating the MEG gain (i.e., lead-field) matrix, which leads to a grid with ~10,000 nodes covering the whole brain. Then, the source grid was combined with the BEM mesh in the MRI coordinate for the BEM forward calculation.
Other conventional MRI sequences typical for identifying structural lesions in TBI participants were also performed: 1) Axial T2*-weighted; 2) Axial fast spin-echo T2-weighted; and 3) Axial FLAIR. The conventional MRIs were carefully reviewed by a Board-certified neuroradiologist (R.R. Lee); no visible lesions were found on the MRI of any participant.
MEG Source Magnitude Imaging using Fast-VESTAL In both 5-minute rs-MEG data sessions with eyes closed, sensor-waveforms were run through a band-pass filter for 1 - 4 Hz (delta band). The data set was then divided into 2.5-second duration epochs, and sensor waveform covariance matrices were calculated for each epoch. A total sensor-waveform covariance matrix for the entire 10-minute recording was calculated by concatenating the covariance matrices from individual epochs. Using the total covariance matrix, voxel-wise MEG slow-wave source magnitude images that cover the whole brain were obtained for each participant following the Fast-VESTAL procedure (Huang et al., 2014a). An Objective Pre-whitening Method was applied to remove correlated environmental noise and objectively select the dominant eigen-modes of sensor-waveform covariance matrix (Huang et al., 2014a).
The Fast-VESTAL technique consists of two steps. First, spatio-temporal L1-minimum-norm MEG source images were obtained for the dominant spatial (i.e., eigen-) modes of sensor-waveform covariance matrix. Next, accurate source time-courses were obtained using an inverse operator constructed from the spatial source images of Step 1. This approach has been successfully used to obtain comprehensive MEG source-magnitude images covering the entire brain for different frequency bands of resting-state brain rhythms (Huang et al., 2014a). The second-order cone programming (SOCP) approach in the minimum L1-norm solver of the SeDuMi software (http://sedumi.ie.lehigh.edu/) was used in the present study. SOCP corrects orientation bias in a one-step approach (Haufe et al., 2011; Ou et al., 2008). The technical details of Fast-VESTAL using the SOCP formulation is in the appendix of our recent paper (C. W. Huang et al., 2016).
Characterizing Abnormal MEG Slow-wave Source Imaging in Individual mTBI Participants The procedure for detecting abnormal MEG slow-wave in single subjects using a voxel-wise approach is detailed in our previous study (Huang et al., 2014b). In the present study, MEG slow-waves in single subjects were evaluated against our voxel-wise normative database that contains 96 healthy individuals between the ages of 18 to 55. The normative database is in MNI-152 atlas coordinates for the MEG source magnitude (spatially smoothed and logarithm transformed). After spatial smoothing and logarithm transformation, the MEG source magnitude images from each mTBI participant were registered to the MNI-152 coordinates, and then converted into Z-score maps using the voxel-wise normative database (Huang et al., 2014b). The abnormal MEG slow-wave generation from each mTBI participant was characterized by the voxels in the Z-score maps with statistical significance (q-value <0.01) after controlling the family-wise error due to multiple comparisons using false discovery rate approach (Benjamini and Hochberg, 1995). We also calculated total abnormal MEG Z-scores by summing up the Z-score from all voxels that showed statistically significant slow-wave generation. The focus of our analyses were on the pre- and post-IASIS change in the abnormal MEG slow-wave generation for both voxel-wise Z-score maps and the total abnormal MEG Z-scores.
ResultsPre- and Post-IASIS Changes in PCS ScoresA key element of the present study was to study clinical symptom changes after IASIS treatment. We found significant reductions of clinical symptoms in the 6 mTBI individuals who participated in the IASIS treatment. Table 2 lists the RPCSQ scores from the pre- and post-IASIS assessments in each participant. PCS total scores across 16 categories were markedly reduced after the IASIS treatment in all participants (Table 2, bottom row; Figure 1A). The observed reduction in PCS total scores between the pre- and post-IASIS assessments was statistically significant (paired group t-test, t = 5.80, p < 0.01, Cohen's d = 2.37)
Pre- and Post-IASIS Changes in Total Abnormal Z-scores from MEG Slow-wave ImagingFig. 1B shows a striking reduction in the total abnormal Z-scores that measured the abnormal MEG slow-wave generation between the pre- and post-IASIS MEG exams. The change in total abnormal MEG Z-scores was also statistically significant (paired group t-test, t = 4.28, p < 0.01, Cohen's d = 1.75)
Next, we correlated the change in MEG slow-waves due to treatment with change in the total PCS scores. In this analysis, we examined two measures: 1) the absolute change of both total abnormal MEG Z-scores and the PCS scores, i.e., pre - post; and 2) the relative change for both total abnormal Z-scores from MEG slow-wave imaging and the PCS scores calculated according to the following formula: (pre - post) / pre. The results showed no significant correlation between absolute change of total abnormal MEG Z-scores and the PCS scores (r = 0.23, p = 0.65). However, relative total abnormal MEG Z-score change significantly correlated with relative total PCS score change. Fig. 1C showed a significant positive correlation between the relative total abnormal MEG Z-score change and relative total PCS score change (r = 0.84, p < 0.05).
Pre- and Post-IASIS Changes in both PCS and MEG Slow-wave Imaging for Individual ParticipantsIn this subsection, detailed information is provided for each participant about pre- and post-IASIS changes related to PCS and abnormal voxel-wise MEG slow-waves. Fig. 2 shows the voxel-wise MEG findings for assessing the effect of IASIS treatment on brain functioning in all 6 mTBI participants. Representative axial slices with major abnormal slow-waves (q < 0.01, FDR correction) are plotted in Fig. 2.
Participant #1: During his tenure in the Marine Corps, Participant #1 experienced an mTBI due to a mortar blast, which caused LOC for over 10 seconds and post-traumatic amnesia for less than 24 hours. Upon entering the study, he reported multiple PCS symptoms persisting since the mortar blast, most notably headache, sleep disturbances, poor memory / poor concentration, and feelings of frustration (Table 2). On the RPCSQ scale, Participant #1’s most notable symptoms were ranked as moderate to severe.
During and following the IASIS treatment, Participant #1 reported that his symptoms greatly abated, going from severe to no problem or mild. His overall RPCSQ score went from 46 to 25, a reduction of 45.7%. The participant also mentioned that he had completely discontinued his use of nicotine after the IASIS treatments, which he claimed was a beneficial result of the treatments. To appreciate the longer lasting effects of the treatment, the participant stated that 6 months after IASIS that the treatment effects had lasted and that he still did not use nicotine.
Compared with pre-IASIS MEG, Participant #1's post-IASIS MEG showed marked reduction of 68.6% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed markedly reduced abnormal slow-waves from frontal pole, posterior cingulate cortex (PCC), right insula, and right hippocampus (Fig. 2). The MEG findings were compatible with reduced PCS for headaches (posterior insular), memory function (related to hippocampus), and feelings of frustration or impatience (frontal pole).
Participant #2: Participant #2 was involved in a multi-vehicle accident that resulted in a loss of consciousness for approximately 10 minutes, which led to mTBI with widespread moderate to severe symptoms. Symptoms included headaches, photophobia, stuttering, overstimulation, anxiety, memory loss, sleep disturbances, and depression. For example, during the initial interview of the study, her photophobia was so severe that we had to turn off all lights in the office to avoid triggering a severe headache.
Halfway through the IASIS sessions, Participant #2 reported a reduction in stuttering, anxiety, headaches, and less visual and auditory overstimulation (particularly reduction of photophobia). She was able go outside and participate in a yard-sale for her church during a sunny afternoon without any headache. After the completion of all IASIS treatments, she reported an even greater reduction of symptoms (i.e., by 50.0%). Her overall score for RPCSQ went from 54 to 27.
In Participant #2, the total abnormal MEG Z-score showed marked reduction of 45.1% in the post-IASIS versus pre-IASIS MEG exams (Figs. 1B and 1C). Specifically, markedly reduced MEG slow-waves were found in anterior cingulate cortex (ACC), right lateral occipital cortex, and right occipital fusiform gyrus (Fig. 2). The MEG findings were also compatible with reduced PCS for headaches (ACC) and photophobia (occipital cortex and fusiform gyrus).
Participant #3: Participant #3 was involved in a driver’s side car accident. The impact caused his head to hit and dent the roof of his car. Prior to the IASIS treatment, he noted severe problems with headaches, forgetfulness / poor memory, poor concentration, taking longer to think, and light sensitivity. He further noted mild problems with noise sensitivity, and irritability, and moderate problems with frustration.
After IASIS, his symptoms drastically reduced from an initial RPCSQ total score of 31 to 15, a reduction of 51.6%. Light sensitivity was reported as only mild whereas headaches, noise sensitivity, irritability, and frustration dropped to insignificant, i.e. no more of a problem. Importantly, severe symptoms became more moderate after treatment, including forgetfulness / poor memory, poor concentration, and taking longer to think.
Compared with the pre-IASIS exam, Participant #3’s post-IASIS MEG exam showed marked reduction of 45.8% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed markedly reduced abnormal slow-waves in PCC, bilateral OFC, and left hippocampus (Fig. 2). The MEG findings are compatible with reduced PCS for memory problems (hippocampus) and headaches (PCC).
Participant #4: While in the Army, Participant #4 reported experiencing an IED blast in which he felt a shock-wave while riding in an Mine-Resistant Ambush Protected vehicle. He was left dazed for a few seconds. Soon after, he had PCS which continued through the first MEG session. Before the IASIS sessions, the participant noted multiple symptoms including noise sensitivity, sleep disturbances, forgetfulness, poor concentration, and taking longer to think. All symptoms were in the mild range in the RPCSQ, with an overall score of 14.
After 3 visits and throughout the remaining IASIS sessions, Participant #4 reported that his quality of sleep had improved, leaving him well rested with a positive change in attitude. Upon finishing all IASIS sessions, this participant recorded an overall score of 1 on the RPCSQ, a reduction of 92.9% in total RPCSQ score. He noted that noise sensitivity was no more of a problem for him. All other symptoms were listed as absent, which meant that Participant #4 was essentially symptom-free.
The pre- and post-IASIS MEG exams show that Participant #4 had a marked reduction of 74.2% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, marked reductions of abnormal slow-waves were found in the right inferior-lateral parietal area / superior temporal gyrus, right hippocampus and amygdala, right inferior temporal pole, and left cerebellum (Fig. 2). The MEG findings were compatible with reduced PCS for noise sensitivity (superior temporal gyrus) and forgetfulness (hippocampus and inferior temporal area).
Participant #5: During his years in the Marine Corp, Participant #5 experienced blunt head trauma while furniture that was being moved struck him in the head. He experienced another blunt head trauma three months prior to starting IASIS when a chair that he was sitting in broke and he hit the back of his head on another chair, intensifying his previous symptoms. Before starting IASIS, Participant #5 listed severe symptoms on the RPCSQ for headaches, noise sensitivity, irritability, frustration, and light sensitivity. Multiple other symptoms such as feelings of dizziness, nausea, sleep disturbance, fatigue, feeling depressed, forgetfulness, having poor concentration, taking longer to think, having blurred vision, and restlessness were scored as moderate, with a total RPCSQ score of 51.
Participant #5 only finished 4 out of the 12 required IASIS treatment sessions. From the beginning, he missed or rescheduled multiple sessions. Because it appeared likely that he might not (actually he did not) finish his IASIS treatment, an MEG exam was performed following his 4th visit, and that MEG was used for this paper. After his 4th IASIS visit, there was a reduction of symptoms but not as remarkable as that of individuals who completed all sessions. His RPSCQ total score changed from 51 in pre-IASIS exam to 45 after the 4th session, with only a marginal reduction of 11.8%. On the RPSCQ, he still noted headaches, irritability, and light sensitivity as severe problems. Similar to pre-IASIS sessions, symptoms of dizziness, sleep disturbance, feeling depressed, forgetfulness, having poor concentration and taking longer to think remained as moderate problems. There were some reductions with noted symptoms of noise sensitivity and feelings of frustration that were reduced from severe to moderate. Experiencing fatigue and restlessness also were reduced from moderate to mild.
The pre-IASIS MEG exams show that Participant #5 had abnormal slow-wave generation from right ACC and PCC, right striatum / insular cortex, right parahippocampus, and left ventro-medial prefrontal cortex (vmPFC) (Fig. 2). In his MEG exam following the 4th IASIS treatment visit, his total abnormal MEG Z-score only showed only a marginal reduction of 12.0% (Figs. 1B and 1C). Specifically, his abnormal slow-wave generation from the right ACC and PCC remained essentially the same. Reduced slow-waves were observed from his right striatum / insular cortex and his right parahippocampus, but increased slow-wave generation was found from his left vmPFC (Fig. 2). The MEG findings were compatible with his persistent PCS at his 4th visit for headache (ACC and PCC) and irritability (vmPFC).
Participant #6: Participant #6, an Army Veteran, experienced an IED while riding in a humvee. He noted being dazed for a few seconds. Before starting IASIS, Participant #6 had a variety of symptoms listed on the RPCSQ with a total score of 31 initially from symptoms ranging as severe, moderate, and mild. The symptoms listed as severe included noise sensitivity and poor concentration; more moderate symptoms included headaches, sleep disturbance, irritability, and taking longer to think, and mild symptoms included fatigue, feeling frustrated, having poor concentration, and light sensitivity.
Throughout the IASIS sessions, Participant #6 noted improvement with sleep quality, accompanied by feeling more energetic. By the end of the sessions, the symptoms listed were all scored as no more of a problem. The total RPCSQ score reduced to 11, a marked reduction of 64.5%.
The pre- and post-IASIS MEG exams show that Participant #6 had a marked reduction of 76.1% in total abnormal MEG Z-score (Figs. 1B and 1C). Specifically, this participant showed marked reduction of abnormal slow-wave generation from the right auditory cortex in superior temporal gyrus as well as right supplementary motor area (SMA) and ACC (Fig. 2). The MEG findings were compatible with reduced PCS for noise sensitivity (auditory cortex in superior temporal gyrus), as well as headaches and poor concentration (SMA and ACC).
DiscussionConsistency with Previous LIP-tES StudiesOur findings that IASIS treatment significantly reduced PCS in participants with mTBI are consistent with TBI studies that used other types of LIP-tES treatment techniques, such as FNS (Larsen et al., 2006; Nelson and Esty, 2015a, 2015b, 2012; Schoenberger et al., 2001). The present study also found significantly reduced abnormal MEG slow-wave generation in mTBI individuals after IASIS. Our finding is compatible with the study by Larsen and colleagues that reported significant decreases in EEG amplitude at the highest amplitude electrode site and at electrode Cz in a mixed population of individuals with TBI and other neurological and/or psychological disorders (Larsen et al., 2006). However, our study demonstrates reduction in abnormal slow-wave generation along with improved PCS after IASIS treatment in individuals with mTBI and documented PCS. Larsen and colleagues also did not specify the range of frequency or frequency band(s) that were altered by LIP-tES treatment. In contrast, our results were directly linked to abnormal MEG slow-waves in delta frequency band (1-4 Hz), which is firmly grounded in neurophysiological studies in animals (Ball et al., 1977; Gloor et al., 1977; Schaul et al., 1978) and subsequently in humans (M. Huang et al., 2016; Huang et al., 2014b, 2012, 2009, Lewine et al., 2007, 1999). Larsen and colleagues' results were also based on analyses in sensor space outside the brain (i.e., EEG electrode positions), whereas our MEG source imaging findings were in image space, inside the brain, and specific to different brain areas. Since an EEG electrode may pick up the signals from multiple brain areas, the signal from one abnormal brain source may contribute to multiple EEG electrodes. By solving the MEG inverse source imaging problem using Fast-VESTAL (Huang et al., 2014a, 2014b), our study is truly a neuroimaging study. In particular, our MEG source imaging approach provides direct measures in the brain regarding the changes of abnormal MEG slow-wave signals following the IASIS treatment (see Fig. 2).
Exploring the Neural Mechanism of IASIS for mTBI Treatment The neural mechanisms underlying IASIS, and LIP-tES in general, are not completely understood (Ochs, 2006). LIP-tES belongs to a large category of transcranial electrical stimulation (tES) techniques that includes transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS) (Abd Hamid et al., 2015; Antal and Herrmann, 2016; Fertonani and Miniussi, 2016). It has been proposed that anodal tDCS and tRNS increase neuronal excitability and may consequently enhance behavioral performance, that cathodal tDCS decreases neuronal excitability and subsequently worsens behavioral performance, and tACS increases neuronal excitability via entrainment of the desired neuronal firing frequency and consequently modulates performance (Paulus, 2011). However, this simplistic, sliding-scale reasoning (from excitation to inhibition or vice versa) does not always explain the results at either the neurophysiological or the behavioral level (Fertonani and Miniussi, 2016). Other models of tES mechanisms include the Stimulation-Dependent, Activity-Dependent, Network Activity-Dependent, Excitation-Inhibition Balance, and Zero-Sum Models, each with its own strengths and limitations (see review in (Fertonani and Miniussi, 2016)). Recently, Fertonani and Miniussi proposed stochastic resonance (SR) as a useful mechanism to explain the general neuromodulation effects of tES (Fertonani and Miniussi, 2016). SR is a phenomenon wherein an endogenous oscillatory brain signal, that is normally too weak to be detected, can be boosted by adding to the signal some noise or input which contains a wide spectrum of frequencies. We believe that the SR model may explain the treatment effects of LIP-tES, including IASIS, for mTBI. In IASIS, the low-intensity KHz pulses contain a wide range of frequencies. When the repetition rate of these pulses is the same as an underlying endogenous oscillatory brain signal, the SR effect may occur. By varying the repetition rate of LIP-tES pulses from low to high, the IASIS process favors a shift in the frequency of endogenous oscillatory brain signals out of the low-frequency (1-4 Hz) range in participants with mTBI. Future research with more participants is needed to test this explanation.
Absolute versus Relative Changes in MEG and PCS Scores Although we found no significant correlation between absolute change in total abnormal MEG Z-scores and total PCS scores, relative change in the total abnormal MEG Z-score was significantly correlated with relative change in the total PCS score of mTBI participants (Fig. 1C). We believe the lack of a significant correlation between the absolute change in MEG and PCS measures was partly due to inter-subject variability in each participant’s criteria for rating the severity of their symptoms. One participant's internal standard for rating the existence and severity of the PCS may be different from that of another participant. Hence, the symptom rating process is subjective to the participant's internal standard. However, as long as such an internal standard remained the same between the baseline and post-IASIS assessments, the relative change scores (i.e., absolute change normalized by the score in the baseline) should be less subjective.
Similarly, the MEG slow-wave generation may be affected by medications that our participants were taking. We did not require the participants to temporarily stop their normal medications. The main advantage of allowing the participants to remain on their medications was to minimize the interruption to their daily life and hence, minimize study attrition. However, some medications may modify MEG slow-wave activity. In particular, neuroleptic sedatives, antidepressants, and hypnotics can globally change brain activity (Niedermeyer and Lopes da Silva, 2005). Thus, an advantage of using relative change in MEG total abnormal Z-scores as a measure of treatment outcome is that it should reduce the effects that medications can have on slow-wave activity. Hence, the strong association between relative changes in MEG abnormal slow-wave activity and relative change in PCS after IASIS in mTBI should not be seriously confounded by medications.
Duration of IASIS Treatment The typical duration of IASIS treatment is 10 weeks (two 30-minute sessions per week) which is based on previous experience from participants with a variety of neurological and/or psychological disorders. Due to study participant availability and resource constraints, we utilized a 6-week, 2 session per week intervention program in the present mTBI study. Noting that Participant #5 only finished 4 out of the 12 treatment sessions and he only showed marginal reductions in PCS and MEG slow-waves. This example shows that dropping off early from the treatment program is not good. On the other hand, prolonging the treatment beyond the necessary duration may not be beneficial for the participant either. Future research is needed to examine the optimal treatment duration of IASIS for mTBI.
Limitations of the Study There are several limitations to the present study. First, the effects of IASIS treatments on neurobehavioral outcomes need to be evaluated using larger mTBI samples. However, despite our small sample size, statistical analyses of pre-post treatment showed large effect sizes suggesting that IASIS treatments are promising for reducing PCS and the generation of abnormal slow waves. Second, future studies using blind and double-blind designs are needed to better validate the efficacy of IASIS and more generally, LIP-tES, for treating mTBI, beyond potential placebo effects. Third, since all participants in the present study were on their standard medical regimens and taking their normal medications, we do not consider this to be a limitation of the study. Nevertheless, studying a group of mTBI individuals who are medication-free or for whom their medication is temporarily withheld would completely remove the medication as a confound in this type of research. However, such a group does not reflect the typical mTBI population and may be extremely difficult to recruit. Lastly, our individual-subject analyses pertaining to possible associations between treatment-related improvements in specific symptoms and regional reductions in abnormal slow-wave generation are qualitative and speculative, owing to the small mTBI sample. Larger samples will be required to quantitatively link regional changes in slow-wave activity with changes in specific PCS in mTBI.
Summary and Conclusions The present study revealed for the first time neuroimaging-based evidence for functional changes in the brain that underlie LIP-tES treatment effects in individuals with mTBI. Abnormal MEG slow-waves were significantly reduced after IASIS treatments in approximately the same brain areas that showed pathological slow-wave generation during the baseline (pre-treatment) MEG exam. Importantly, PCS were also typically reduced, or even eliminated altogether, following IASIS treatments. Furthermore, relative reductions of MEG slow-wave total abnormal Z-score correlated strongly with the relative reduction of the PCS score. The information of the loci that generate abnormal MEG slow waves in each mTBI individual may also be used as a guide in developing an optimal and subject-specific IASIS treatment plan for that individual. Altogether, the present study sets the stage for a new avenue of research that can advance an understanding of the mechanisms underlying low-intensity transcranial stimulation and its relevance to behavioral outcomes that significantly impact the quality of life in individuals with mTBI.
AcknowledgmentsThis work was supported in part by Merit Review Grants from the Department of Veterans Affairs to M.X. Huang (I01-CX000499, MHBA-010-14F, NURC-022-10F, NEUC-044-06S) and R.R. Lee.
DisclaimersAlthough Mr. Barry Bruder and Mr. Corey Snook are associated with IASIS Technologies, Inc., and Mind-Brain Training Institute, respectively, their contributions to this work were limited to providing to the UCSD group training, technical support, and technical information related to the IASIS system. Neither of them was involved in recruiting participants, data acquisition, or data analysis in the present study.
Fig. 1: A: Significant reduction in total Rivermead PCS scores between pre- and post-IASIS assessments (p<0.01). B: Significant reduction in total abnormal Z-score MEG slow-wave source imaging between pre- and post-IASIS MEG exams (p<0.01). C: Relative change in total abnormal MEG Z-score correlates with relative change in total PCS score (p<0.05). Symbols "D", "O", "×", "+", "", and "à" represent Participants #1, #2, ..., and #6, respectively.
Fig. 2: Changes in abnormal MEG slow-waves between pre- and post-IASIS MEG exams in six mTBI participants. Green arrows indicate areas with abnormal slow-waves at the baseline but markedly reduced (> 30%) after IASIS. Red arrow indicates an area with markedly increased (> 30%) slow-wave after IASIS in mTBI Participant #5, who completed only 4 of 12 scheduled sessions. The hot spots without arrows indicate abnormal slow waves that did not show marked change (< 30%) after IASIS treatment.
ReferencesAbd Hamid, A.I., Gall, C., Speck, O., Antal, A., Sabel, B.A., 2015. Effects of alternating current stimulation on the healthy and diseased brain. Front. Neurosci. 9, 391. doi:10.3389/fnins.2015.00391
Alexander, M.P., 1995. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45, 1253–1260.
Antal, A., Herrmann, C.S., 2016. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast. 2016, 3616807. doi:10.1155/2016/3616807
Ball, G.J., Gloor, P., Schaul, N., 1977. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr.Clin.Neurophysiol. 43, 346–361.
Benjamini, Y., Hochberg, Y., 1995. Controlling the false positive rate: a prectical and powerful approach to multiple testing. JRStatSocSeries B 57, 289–300.
Bigler, E.D., 2008. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 14, 1–22.
Binder, L.M., 1997. A review of mild head trauma. Part II: Clinical implications. J.Clin.Exp.Neuropsychol. 19, 432–457.
Binder, L.M., 1986. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J.Clin.Exp.Neuropsychol. 8, 323–346.
Bohnen, N., Jolles, J., Twijnstra, A., 1992. Neuropsychological deficits in patients with persistent symptoms six months after mild head injury. Neurosurgery 30, 692–695.
Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2003. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Centers for Disease Control and Prevention, Atlanta, GA, USA.
Cohen, D., Schlapfer, U., Ahlfors, S., Hamalainen, M., Halgren, E., 2002. New Six-Layer Magnetically-Shielded Room for MEG, in: Nowak, H.H.J., Giebler, F. (Eds.), Proceedings of the 13th International Conference on Biomagnetism. VDE Verlag, Jena, Germany, pp. 919–921.
Cooper, D.B., Bunner, A.E., Kennedy, J.E., Balldin, V., Tate, D.F., Eapen, B.C., and Jaramillo, C.A., 2015. Treatment of persistent post-concussive symptoms after mild traumatic brain injury: a systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging Behav. 9, 403–420.
Dikmen, S.S., Ross, B.L., Machamer, J.E., and Temkin, N.R., 1995. One year psychosocial outcome in head injury. J. Int. Neuropsychol. Soc. 1, 67–77.
Fertonani, A., Miniussi, C., 2016. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. doi:10.1177/1073858416631966
Gloor, P., Ball, G., Schaul, N., 1977. Brain lesions that produce delta waves in the EEG. Neurology 27, 326–333.
Haufe, S., Tomioka, R., Dickhaus, T., Sannelli, C., Blankertz, B., Nolte, G., Muller, K.R., 2011. Large-scale EEG/MEG source localization with spatial flexibility. Neuroimage. 54, 851–859.
Huang, C.W., Huang, M.-X., Ji, Z., Swan, A.R., Angeles, A.M., Song, T., Huang, J.W., Lee, R.R., 2016. High-resolution MEG source imaging approach to accurately localize Broca’s area in patients with brain tumor or epilepsy. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 127, 2308–2316. doi:10.1016/j.clinph.2016.02.007
Huang, M., Risling, M., Baker, D.G., 2016. The role of biomarkers and MEG-based imaging markers in the diagnosis of post-traumatic stress disorder and blast-induced mild traumatic brain injury. Psychoneuroendocrinology 63, 398–409. doi:10.1016/j.psyneuen.2015.02.008
Huang, M.-X., Huang, C.W., Robb, A., Angeles, A., Nichols, S.L., Baker, D.G., Song, T., Harrington, D.L., Theilmann, R.J., Srinivasan, R., Heister, D., Diwakar, M., Canive, J.M., Edgar, J.C., Chen, Y.-H., Ji, Z., Shen, M., El-Gabalawy, F., Levy, M., McLay, R., Webb-Murphy, J., Liu, T.T., Drake, A., Lee, R.R., 2014a. MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. NeuroImage 84, 585–604. doi:10.1016/j.neuroimage.2013.09.022
Huang, M.-X., Nichols, S., Baker, D.G., Robb, A., Angeles, A., Yurgil, K.A., Drake, A., Levy, M., Song, T., McLay, R., Theilmann, R.J., Diwakar, M., Risbrough, V.B., Ji, Z., Huang, C.W., Chang, D.G., Harrington, D.L., Muzzatti, L., Canive, J.M., Christopher Edgar, J., Chen, Y.-H., Lee, R.R., 2014b. Single-subject-based whole-brain MEG slow-wave imaging approach for detecting abnormality in patients with mild traumatic brain injury. NeuroImage Clin. 5, 109–119. doi:10.1016/j.nicl.2014.06.004
Huang, M.-X., Nichols, S., Robb, A., Angeles, A., Drake, A., Holland, M., Asmussen, S., D’Andrea, J., Chun, W., Levy, M., Cui, L., Song, T., Baker, D.G., Hammer, P., McLay, R., Theilmann, R.J., Coimbra, R., Diwakar, M., Boyd, C., Neff, J., Liu, T.T., Webb-Murphy, J., Farinpour, R., Cheung, C., Harrington, D.L., Heister, D., Lee, R.R., 2012. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. NeuroImage 61, 1067–1082. doi:10.1016/j.neuroimage.2012.04.029
Huang, M.X., Song, T., Hagler, D.J., Jr., Podgorny, I., Jousmaki, V., Cui, L., Gaa, K., Harrington, D.L., Dale, A.M., Lee, R.R., Elman, J., Halgren, E., 2007. A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage 37, 731–748.
Huang, M.X., Theilmann, R.J., Robb, A., Angeles, A., Nichols, S., Drake, A., D’Andrea, J., Levy, M., Holland, M., Song, T., Ge, S., Hwang, E., Yoo, K., Cui, L., Baker, D.G., Trauner, D., Coimbra, R., and Lee, R.R., 2009. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26, 1213–1226.
Hyvarinen, A., 1999. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 10, 626–634.
Hyvarinen, A., and Oja, E., 2000. Independent component analysis: algorithms and applications. Neural. Netw. 13, 411–430.
Jeter, C.B., Hergenroeder, G.W., Hylin, M.J., Redell, J.B., Moore, A.N., and Dash, P.K., 2013. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma 30, 657–670.
Jovicich, J., Czanner, S., Greve, D., Haley, E., van der, K.A., Gollub, R., Kennedy, D., Schmitt, F., Brown, G., Macfall, J., Fischl, B., Dale, A., 2006. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 30, 436–443.
King, N.S., Crawford, S., Wenden, F.J., Moss, N.E., Wade, D.T., 1995. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242, 587–592.
Larsen, S., Harrington, K., Hicks, S., 2006. The LENS (Low Energy Neurofeedback System): A Clinical Outcomes Study on One Hundred Patients at Stone Mountain Center, New York. J. Neurother. 10, 69–78. doi:10.1300/J184v10n02_06
Leahy, R.M., Mosher, J.C., Spencer, M.E., Huang, M.X., and Lewine, J.D., 1998. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr Clin. Neurophysiol. 107, 159–173.
Lewine, J.D., Davis, J.T., Bigler, E.D., Thoma, R., Hill, D., Funke, M., Sloan, J.H., Hall, S., and Orrison, W.W., 2007. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 22, 141–155.
Lewine, J.D., Davis, J.T., Sloan, J.H., Kodituwakku, P.W., and Orrison, W.W., Jr., 1999. Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR Am. J. Neuroradiol. 20, 857–866.
MacGregor, A.J., Dougherty, A.L., and Galarneau, M.R., 2011. Injury-Specific Correlates of Combat-Related Traumatic Brain Injury in Operation Iraqi Freedom. J. Head Trauma Rehabil. 26, 312–318.
McCauley, S.R., Wilde, E.A., Miller, E.R., Frisby, M.L., Garza, H.M., Varghese, R., Levin, H.S., Robertson, C.S., McCarthy, J.J., 2013. Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J. Neurotrauma 30, 642–652. doi:10.1089/neu.2012.2393
Morissette, S.B., Woodward, M., Kimbrel, N.A., Meyer, E.C., Kruse, M.I., Dolan, S., and Gulliver, S.B., 2011. Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabil. Psychol. 56, 340–350.
Mosher, J.C., Leahy, R.M., and Lewis, P.S., 1999. EEG and MEG: forward solutions for inverse methods. IEEE Trans. Biomed. Eng. 46, 245–259.
Nelson, D.V., Esty, M.L., 2015a. Neurotherapy of Traumatic Brain Injury/Post-Traumatic Stress Symptoms in Vietnam Veterans. Mil. Med. 180, e1111-1114. doi:10.7205/MILMED-D-14-00696
Nelson, D.V., Esty, M.L., 2015b. Neurotherapy for chronic headache following traumatic brain injury. Mil. Med. Res. 2, 22. doi:10.1186/s40779-015-0049-y
Nelson, D.V., Esty, M.L., 2012. Neurotherapy of traumatic brain injury/posttraumatic stress symptoms in OEF/OIF veterans. J. Neuropsychiatry Clin. Neurosci. 24, 237–240. doi:10.1176/appi.neuropsych.11020041
Niedermeyer, E., Lopes da Silva, F.H., 2005. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins, Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo.
Ochs, L., 2006. The Low Energy Neurofeedback System (LENS): Theory, Background, and Introduction. J. Neurother. 10, 5–39. doi:10.1300/J184v10n02_02
Ochs, L., 1997. Flexyx Neurotherapy System Operating Manual for the J&J USE2 and the I-330 ompact 2C EEG.
Ou, W., Golland, P., and Hamalainen, M., 2008. A distributed spatio-temporal EEG/MEG inverse solver. Med. Image Comput. Comput. Assist. Interv. 11, 26–34.
Paulus, W., 2011. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21, 602–617. doi:10.1080/09602011.2011.557292
Rimel, R.W., Giordani, B., Barth, J.T., Boll, T.J., Jane, J.A., 1981. Disability caused by minor head injury. Neurosurgery 9, 221–228.
Robb Swan, A., Nichols, S., Drake, A., Angeles, A., Diwakar, M., Song, T., Lee, R.R., Huang, M.-X., 2015. Magnetoencephalography Slow-Wave Detection in Patients with Mild Traumatic Brain Injury and Ongoing Symptoms Correlated with Long-Term Neuropsychological Outcome. J. Neurotrauma 32, 1510–1521. doi:10.1089/neu.2014.3654
Rutherford, W.H., 1989. Postconcussion symptoms: Relationship to acute neurological indices, individual differences, and circumstances of injury, in: Levin, H., Eisenberg, H., Benton, A.L. (Eds.), Mild Head Injury. Oxford University Press, New York, pp. 217–228.
Schaul, N., Gloor, P., Ball, G., Gotman, J., 1978. The electromicrophysiology of delta waves induced by systemic atropine. Brain Res 143, 475–486.
Schneiderman, A.I., Braver, E.R., and Kang, H.K., 2008. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 167, 1446–1452.
Schoenberger, N.E., Shif, S.C., Esty, M.L., Ochs, L., Matheis, R.J., 2001. Flexyx Neurotherapy System in the treatment of traumatic brain injury: an initial evaluation. J. Head Trauma Rehabil. 16, 260–274.
Shen, X., Tokoglu, F., Papademetris, X., Constable, R.T., 2013. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 82, 403–415.
Snook, C., 2013. US Patent Application for METHOD AND SYSTEM FOR RETRAINING BRAINWAVE PATTERNS USING ULTRA LOW POWER DIRECT ELECTRICAL STIMULATION FEEDBACK Patent Application (Application #20140330157 issued November 6, 2014) - Justia Patents Search [WWW Document]. URL http://patents.justia.com/patent/20140330157 (accessed 10.9.16).
Song, T., Gaa, K., Cui, L., Feffer, L., Lee, R.R., and Huang, M., 2008. Evaluation of signal space separation via simulation. Med. Biol. Eng. Comput. 46, 923–932.
Taulu, S., Kajola, M., and Simola, J., 2004. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 16, 269–275.
Taulu, S., Simola, J., and Kajola, M., 2004. MEG recordings of DC fields using the signal space separation method (SSS). Neurol. Clin. Neurophysiol. 2004, 35.
Teasdale, G., Jennett, B., 1974. Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84.
Terrio, H., Brenner, L.A., Ivins, B.J., Cho, J.M., Helmick, K., Schwab, K., Scally, K., Bretthauer, R., and Warden, D., 2009. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23.
The Management of Concussion/mTBI Working Group. 2009. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 46, CP1-68.
Alexander, M.P., 1995. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45, 1253–1260.
Antal, A., Herrmann, C.S., 2016. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast. 2016, 3616807. doi:10.1155/2016/3616807
Ball, G.J., Gloor, P., Schaul, N., 1977. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr.Clin.Neurophysiol. 43, 346–361.
Benjamini, Y., Hochberg, Y., 1995. Controlling the false positive rate: a prectical and powerful approach to multiple testing. JRStatSocSeries B 57, 289–300.
Bigler, E.D., 2008. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 14, 1–22.
Binder, L.M., 1997. A review of mild head trauma. Part II: Clinical implications. J.Clin.Exp.Neuropsychol. 19, 432–457.
Binder, L.M., 1986. Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J.Clin.Exp.Neuropsychol. 8, 323–346.
Bohnen, N., Jolles, J., Twijnstra, A., 1992. Neuropsychological deficits in patients with persistent symptoms six months after mild head injury. Neurosurgery 30, 692–695.
Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2003. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Centers for Disease Control and Prevention, Atlanta, GA, USA.
Cohen, D., Schlapfer, U., Ahlfors, S., Hamalainen, M., Halgren, E., 2002. New Six-Layer Magnetically-Shielded Room for MEG, in: Nowak, H.H.J., Giebler, F. (Eds.), Proceedings of the 13th International Conference on Biomagnetism. VDE Verlag, Jena, Germany, pp. 919–921.
Cooper, D.B., Bunner, A.E., Kennedy, J.E., Balldin, V., Tate, D.F., Eapen, B.C., and Jaramillo, C.A., 2015. Treatment of persistent post-concussive symptoms after mild traumatic brain injury: a systematic review of cognitive rehabilitation and behavioral health interventions in military service members and veterans. Brain Imaging Behav. 9, 403–420.
Dikmen, S.S., Ross, B.L., Machamer, J.E., and Temkin, N.R., 1995. One year psychosocial outcome in head injury. J. Int. Neuropsychol. Soc. 1, 67–77.
Fertonani, A., Miniussi, C., 2016. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry. doi:10.1177/1073858416631966
Gloor, P., Ball, G., Schaul, N., 1977. Brain lesions that produce delta waves in the EEG. Neurology 27, 326–333.
Haufe, S., Tomioka, R., Dickhaus, T., Sannelli, C., Blankertz, B., Nolte, G., Muller, K.R., 2011. Large-scale EEG/MEG source localization with spatial flexibility. Neuroimage. 54, 851–859.
Huang, C.W., Huang, M.-X., Ji, Z., Swan, A.R., Angeles, A.M., Song, T., Huang, J.W., Lee, R.R., 2016. High-resolution MEG source imaging approach to accurately localize Broca’s area in patients with brain tumor or epilepsy. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 127, 2308–2316. doi:10.1016/j.clinph.2016.02.007
Huang, M., Risling, M., Baker, D.G., 2016. The role of biomarkers and MEG-based imaging markers in the diagnosis of post-traumatic stress disorder and blast-induced mild traumatic brain injury. Psychoneuroendocrinology 63, 398–409. doi:10.1016/j.psyneuen.2015.02.008
Huang, M.-X., Huang, C.W., Robb, A., Angeles, A., Nichols, S.L., Baker, D.G., Song, T., Harrington, D.L., Theilmann, R.J., Srinivasan, R., Heister, D., Diwakar, M., Canive, J.M., Edgar, J.C., Chen, Y.-H., Ji, Z., Shen, M., El-Gabalawy, F., Levy, M., McLay, R., Webb-Murphy, J., Liu, T.T., Drake, A., Lee, R.R., 2014a. MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images. NeuroImage 84, 585–604. doi:10.1016/j.neuroimage.2013.09.022
Huang, M.-X., Nichols, S., Baker, D.G., Robb, A., Angeles, A., Yurgil, K.A., Drake, A., Levy, M., Song, T., McLay, R., Theilmann, R.J., Diwakar, M., Risbrough, V.B., Ji, Z., Huang, C.W., Chang, D.G., Harrington, D.L., Muzzatti, L., Canive, J.M., Christopher Edgar, J., Chen, Y.-H., Lee, R.R., 2014b. Single-subject-based whole-brain MEG slow-wave imaging approach for detecting abnormality in patients with mild traumatic brain injury. NeuroImage Clin. 5, 109–119. doi:10.1016/j.nicl.2014.06.004
Huang, M.-X., Nichols, S., Robb, A., Angeles, A., Drake, A., Holland, M., Asmussen, S., D’Andrea, J., Chun, W., Levy, M., Cui, L., Song, T., Baker, D.G., Hammer, P., McLay, R., Theilmann, R.J., Coimbra, R., Diwakar, M., Boyd, C., Neff, J., Liu, T.T., Webb-Murphy, J., Farinpour, R., Cheung, C., Harrington, D.L., Heister, D., Lee, R.R., 2012. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. NeuroImage 61, 1067–1082. doi:10.1016/j.neuroimage.2012.04.029
Huang, M.X., Song, T., Hagler, D.J., Jr., Podgorny, I., Jousmaki, V., Cui, L., Gaa, K., Harrington, D.L., Dale, A.M., Lee, R.R., Elman, J., Halgren, E., 2007. A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage 37, 731–748.
Huang, M.X., Theilmann, R.J., Robb, A., Angeles, A., Nichols, S., Drake, A., D’Andrea, J., Levy, M., Holland, M., Song, T., Ge, S., Hwang, E., Yoo, K., Cui, L., Baker, D.G., Trauner, D., Coimbra, R., and Lee, R.R., 2009. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma 26, 1213–1226.
Hyvarinen, A., 1999. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 10, 626–634.
Hyvarinen, A., and Oja, E., 2000. Independent component analysis: algorithms and applications. Neural. Netw. 13, 411–430.
Jeter, C.B., Hergenroeder, G.W., Hylin, M.J., Redell, J.B., Moore, A.N., and Dash, P.K., 2013. Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J. Neurotrauma 30, 657–670.
Jovicich, J., Czanner, S., Greve, D., Haley, E., van der, K.A., Gollub, R., Kennedy, D., Schmitt, F., Brown, G., Macfall, J., Fischl, B., Dale, A., 2006. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 30, 436–443.
King, N.S., Crawford, S., Wenden, F.J., Moss, N.E., Wade, D.T., 1995. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242, 587–592.
Larsen, S., Harrington, K., Hicks, S., 2006. The LENS (Low Energy Neurofeedback System): A Clinical Outcomes Study on One Hundred Patients at Stone Mountain Center, New York. J. Neurother. 10, 69–78. doi:10.1300/J184v10n02_06
Leahy, R.M., Mosher, J.C., Spencer, M.E., Huang, M.X., and Lewine, J.D., 1998. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr Clin. Neurophysiol. 107, 159–173.
Lewine, J.D., Davis, J.T., Bigler, E.D., Thoma, R., Hill, D., Funke, M., Sloan, J.H., Hall, S., and Orrison, W.W., 2007. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT, and MRI. J. Head Trauma Rehabil. 22, 141–155.
Lewine, J.D., Davis, J.T., Sloan, J.H., Kodituwakku, P.W., and Orrison, W.W., Jr., 1999. Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. AJNR Am. J. Neuroradiol. 20, 857–866.
MacGregor, A.J., Dougherty, A.L., and Galarneau, M.R., 2011. Injury-Specific Correlates of Combat-Related Traumatic Brain Injury in Operation Iraqi Freedom. J. Head Trauma Rehabil. 26, 312–318.
McCauley, S.R., Wilde, E.A., Miller, E.R., Frisby, M.L., Garza, H.M., Varghese, R., Levin, H.S., Robertson, C.S., McCarthy, J.J., 2013. Preinjury resilience and mood as predictors of early outcome following mild traumatic brain injury. J. Neurotrauma 30, 642–652. doi:10.1089/neu.2012.2393
Morissette, S.B., Woodward, M., Kimbrel, N.A., Meyer, E.C., Kruse, M.I., Dolan, S., and Gulliver, S.B., 2011. Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabil. Psychol. 56, 340–350.
Mosher, J.C., Leahy, R.M., and Lewis, P.S., 1999. EEG and MEG: forward solutions for inverse methods. IEEE Trans. Biomed. Eng. 46, 245–259.
Nelson, D.V., Esty, M.L., 2015a. Neurotherapy of Traumatic Brain Injury/Post-Traumatic Stress Symptoms in Vietnam Veterans. Mil. Med. 180, e1111-1114. doi:10.7205/MILMED-D-14-00696
Nelson, D.V., Esty, M.L., 2015b. Neurotherapy for chronic headache following traumatic brain injury. Mil. Med. Res. 2, 22. doi:10.1186/s40779-015-0049-y
Nelson, D.V., Esty, M.L., 2012. Neurotherapy of traumatic brain injury/posttraumatic stress symptoms in OEF/OIF veterans. J. Neuropsychiatry Clin. Neurosci. 24, 237–240. doi:10.1176/appi.neuropsych.11020041
Niedermeyer, E., Lopes da Silva, F.H., 2005. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins, Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo.
Ochs, L., 2006. The Low Energy Neurofeedback System (LENS): Theory, Background, and Introduction. J. Neurother. 10, 5–39. doi:10.1300/J184v10n02_02
Ochs, L., 1997. Flexyx Neurotherapy System Operating Manual for the J&J USE2 and the I-330 ompact 2C EEG.
Ou, W., Golland, P., and Hamalainen, M., 2008. A distributed spatio-temporal EEG/MEG inverse solver. Med. Image Comput. Comput. Assist. Interv. 11, 26–34.
Paulus, W., 2011. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21, 602–617. doi:10.1080/09602011.2011.557292
Rimel, R.W., Giordani, B., Barth, J.T., Boll, T.J., Jane, J.A., 1981. Disability caused by minor head injury. Neurosurgery 9, 221–228.
Robb Swan, A., Nichols, S., Drake, A., Angeles, A., Diwakar, M., Song, T., Lee, R.R., Huang, M.-X., 2015. Magnetoencephalography Slow-Wave Detection in Patients with Mild Traumatic Brain Injury and Ongoing Symptoms Correlated with Long-Term Neuropsychological Outcome. J. Neurotrauma 32, 1510–1521. doi:10.1089/neu.2014.3654
Rutherford, W.H., 1989. Postconcussion symptoms: Relationship to acute neurological indices, individual differences, and circumstances of injury, in: Levin, H., Eisenberg, H., Benton, A.L. (Eds.), Mild Head Injury. Oxford University Press, New York, pp. 217–228.
Schaul, N., Gloor, P., Ball, G., Gotman, J., 1978. The electromicrophysiology of delta waves induced by systemic atropine. Brain Res 143, 475–486.
Schneiderman, A.I., Braver, E.R., and Kang, H.K., 2008. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 167, 1446–1452.
Schoenberger, N.E., Shif, S.C., Esty, M.L., Ochs, L., Matheis, R.J., 2001. Flexyx Neurotherapy System in the treatment of traumatic brain injury: an initial evaluation. J. Head Trauma Rehabil. 16, 260–274.
Shen, X., Tokoglu, F., Papademetris, X., Constable, R.T., 2013. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 82, 403–415.
Snook, C., 2013. US Patent Application for METHOD AND SYSTEM FOR RETRAINING BRAINWAVE PATTERNS USING ULTRA LOW POWER DIRECT ELECTRICAL STIMULATION FEEDBACK Patent Application (Application #20140330157 issued November 6, 2014) - Justia Patents Search [WWW Document]. URL http://patents.justia.com/patent/20140330157 (accessed 10.9.16).
Song, T., Gaa, K., Cui, L., Feffer, L., Lee, R.R., and Huang, M., 2008. Evaluation of signal space separation via simulation. Med. Biol. Eng. Comput. 46, 923–932.
Taulu, S., Kajola, M., and Simola, J., 2004. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 16, 269–275.
Taulu, S., Simola, J., and Kajola, M., 2004. MEG recordings of DC fields using the signal space separation method (SSS). Neurol. Clin. Neurophysiol. 2004, 35.
Teasdale, G., Jennett, B., 1974. Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84.
Terrio, H., Brenner, L.A., Ivins, B.J., Cho, J.M., Helmick, K., Schwab, K., Scally, K., Bretthauer, R., and Warden, D., 2009. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 24, 14–23.
The Management of Concussion/mTBI Working Group. 2009. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 46, CP1-68.